Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey
- PMID: 22053109
- PMCID: PMC3265196
- DOI: 10.1182/blood-2011-04-325225
Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey
Abstract
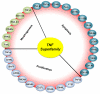
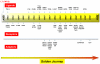
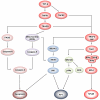
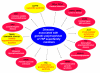
Although activity that induced tumor regression was observed and termed tumor necrosis factor (TNF) as early as the 1960s, the true identity of TNF was not clear until 1984, when Aggarwal and coworkers reported, for the first time, the isolation of 2 cytotoxic factors: one, derived from macrophages (molecular mass 17 kDa), was named TNF, and the second, derived from lymphocytes (20 kDa), was named lymphotoxin. Because the 2 cytotoxic factors exhibited 50% amino acid sequence homology and bound to the same receptor, they came to be called TNF-α and TNF-β. Identification of the protein sequences led to cloning of their cDNA. Based on sequence homology to TNF-α, now a total of 19 members of the TNF superfamily have been identified, along with 29 interacting receptors, and several molecules that interact with the cytoplasmic domain of these receptors. The roles of the TNF superfamily in inflammation, apoptosis, proliferation, invasion, angiogenesis, metastasis, and morphogenesis have been documented. Their roles in immunologic, cardiovascular, neurologic, pulmonary, and metabolic diseases are becoming apparent. TNF superfamily members are active targets for drug development, as indicated by the recent approval and expanding market of TNF blockers used to treat rheumatoid arthritis, psoriasis, Crohns disease, and osteoporosis, with a total market of more than US $20 billion. As we learn more about this family, more therapeutics will probably emerge. In this review, we summarize the initial discovery of TNF-α, and the insights gained regarding the roles of this molecule and its related family members in normal physiology and disease.
Figures


References
-
- Bhardwaj A, Aggarwal BB. Receptor-mediated choreography of life and death. J Clin Immunol. 2003;23(5):317–332. - PubMed
-
- Gaur U, Aggarwal BB. Regulation of proliferation, survival and apoptosis by members of the TNF superfamily. Biochem Pharmacol. 2003;66(8):1403–1408. - PubMed
-
- Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3(9):745–756. - PubMed
-
- O'Malley Action of bacterial polysaccharide on tumors: II. Damage of Sarcoma 37 by serum of mice treated with Serratia marcescens polysaccharide, and induced tolerance. J Natl Cancer Inst. 1962;29:1169–1175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

