The sodium channel as a target for local anesthetic drugs
- PMID: 22053156
- PMCID: PMC3205381
- DOI: 10.3389/fphar.2011.00068
The sodium channel as a target for local anesthetic drugs
Abstract
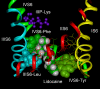
Na channels are the source of excitatory currents for the nervous system and muscle. They are the target for a class of drugs called local anesthetics (LA), which have been used for local and regional anesthesia and for excitatory problems such as epilepsy and cardiac arrhythmia. These drugs are prototypes for new analgesic drugs. The drug-binding site has been localized to the inner pore of the channel, where drugs interact mainly with a phenylalanine in domain IV S6. Drug affinity is both voltage- and use-dependent. Voltage-dependency is the result of changes in the conformation of the inner pore during channel activation and opening, allowing high energy interaction of drugs with the phenylalanine. LA drugs also reduce the gating current of Na channels, which represents the movement of charged residues in the voltage sensors. Specifically, drug binding to phenylalanine locks the domain III S4 in its outward (activated) position, and slows recovery of the domain IV S4. Although strongly affecting gating, LA drugs almost certainly also block by steric occlusion of the pore. Molecular definition of the binding and blocking interactions may help in new drug development.
Keywords: Na channel; gating currents; lidocaine; local anesthetics; molecular modeling.
Figures
References
-
- Alpert L. A., Fozzard H. A., Hanck D. A., Makielski J. C. (1989). Is there a second external lidocaine binding site on mammalian cardiac cells? Am. J. Physiol. 257, H79–H84 - PubMed
-
- Bennett P. B., Valenzuela C., Chen L.-Q., Kallen R. G. (1995). On the molecular nature of the lidocaine receptor of cardiac Na channels: modification of block by alterations in α-subunit III-IV interdomain. Circ. Res. 7, 584–592 - PubMed
-
- Bezanilla F. (2000). The voltage sensor in voltage dependent ion channels. Physiol. Rev. 80, 555–592 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

