Overcoming memory T-cell responses for induction of delayed tolerance in nonhuman primates
- PMID: 22053723
- PMCID: PMC3268945
- DOI: 10.1111/j.1600-6143.2011.03795.x
Overcoming memory T-cell responses for induction of delayed tolerance in nonhuman primates
Abstract
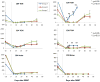
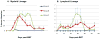
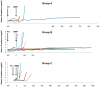
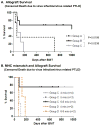
The presence of alloreactive memory T cells is a major barrier for induction of tolerance in primates. In theory, delaying conditioning for tolerance induction until after organ transplantation could further decrease the efficacy of the regimen, since preexisting alloreactive memory T cells might be stimulated by the transplanted organ. Here, we show that such "delayed tolerance" can be induced in nonhuman primates through the mixed chimerism approach, if specific modifications to overcome/avoid donor-specific memory T-cell responses are provided. These modifications include adequate depletion of CD8+ memory T cells and timing of donor bone marrow administration to minimize levels of proinflammatory cytokines. Using this modified approach, mixed chimerism was induced successfully in 11 of 13 recipients of previously placed renal allografts and long-term survival without immunosuppression could be achieved in at least 6 of these 11 animals.
© 2011 The American Society of Transplantation and the American Society of Transplant Surgeons.
Conflict of interest statement
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation. This manuscript was also not prepared or funded by any commercial organization.
Figures





Comment in
-
Tolerance induction after organ transplantation, "delayed tolerance," via the mixed chimerism approach: planting flowers in a battle field.Chimerism. 2012 Jan-Mar;3(1):24-8. doi: 10.4161/chim.20096. Chimerism. 2012. PMID: 22690270 Free PMC article.
Similar articles
-
Depletion of CD8 memory T cells for induction of tolerance of a previously transplanted kidney allograft.Am J Transplant. 2007 May;7(5):1055-61. doi: 10.1111/j.1600-6143.2006.01703.x. Epub 2007 Feb 7. Am J Transplant. 2007. PMID: 17286617 Free PMC article.
-
Alefacept promotes immunosuppression-free renal allograft survival in nonhuman primates via depletion of recipient memory T cells.Am J Transplant. 2013 Dec;13(12):3223-9. doi: 10.1111/ajt.12500. Epub 2013 Oct 24. Am J Transplant. 2013. PMID: 24165326 Free PMC article.
-
Host alloreactive memory T cells influence tolerance to kidney allografts in nonhuman primates.Sci Transl Med. 2011 Jun 8;3(86):86ra51. doi: 10.1126/scitranslmed.3002093. Sci Transl Med. 2011. PMID: 21653831 Free PMC article.
-
Preclinical and clinical studies on the induction of renal allograft tolerance through transient mixed chimerism.Curr Opin Organ Transplant. 2011 Aug;16(4):366-71. doi: 10.1097/MOT.0b013e3283484b2c. Curr Opin Organ Transplant. 2011. PMID: 21666482 Free PMC article. Review.
-
Tolerance induction: hematopoietic chimerism.Curr Opin Organ Transplant. 2013 Aug;18(4):402-7. doi: 10.1097/MOT.0b013e328363621d. Curr Opin Organ Transplant. 2013. PMID: 23838644 Review.
Cited by
-
Long-term Kinetics of Intragraft Gene Signatures in Renal Allograft Tolerance Induced by Transient Mixed Chimerism.Transplantation. 2019 Nov;103(11):e334-e344. doi: 10.1097/TP.0000000000002911. Transplantation. 2019. PMID: 31397805 Free PMC article.
-
Transplantation tolerance and its outcome during infections and inflammation.Immunol Rev. 2014 Mar;258(1):80-101. doi: 10.1111/imr.12147. Immunol Rev. 2014. PMID: 24517427 Free PMC article. Review.
-
New frontiers in immunosuppression.J Thorac Dis. 2018 May;10(5):3141-3155. doi: 10.21037/jtd.2018.04.79. J Thorac Dis. 2018. PMID: 29997983 Free PMC article. Review.
-
Chimerism-based tolerance in organ transplantation: preclinical and clinical studies.Clin Exp Immunol. 2017 Aug;189(2):190-196. doi: 10.1111/cei.12969. Epub 2017 Apr 20. Clin Exp Immunol. 2017. PMID: 28369830 Free PMC article. Review.
-
Hematopoietic stem cell infusion/transplantation for induction of allograft tolerance.Curr Opin Organ Transplant. 2015 Feb;20(1):49-56. doi: 10.1097/MOT.0000000000000159. Curr Opin Organ Transplant. 2015. PMID: 25563992 Free PMC article. Review.
References
-
- Chen HD, Fraire AE, Joris I, Brehm MA, Welsh RM, Selin LK. Memory CD8+ T cells in heterologous antiviral immunity and immunopathology in the lung. Nat Immunol. 2001;2(11):1067–1076. - PubMed
-
- Welsh RM, Selin LK. No one is naïve: the significance of heterologous T-cell immunity. Nat Rev Immunol. 2002;2(6):417–426. - PubMed
Publication types
MeSH terms
Grants and funding
- 5R01 AI50987-03/AI/NIAID NIH HHS/United States
- U01 AI094374/AI/NIAID NIH HHS/United States
- U19 AI102405/AI/NIAID NIH HHS/United States
- P0I-HL18646/HL/NHLBI NIH HHS/United States
- MR/J006742/1/MRC_/Medical Research Council/United Kingdom
- R21 AI037692/AI/NIAID NIH HHS/United States
- R01 AI050987/AI/NIAID NIH HHS/United States
- P01 HL018646/HL/NHLBI NIH HHS/United States
- U01 AI102427/AI/NIAID NIH HHS/United States
- R00000000008108/PHS HHS/United States
- R0IA137692/PHS HHS/United States
- R01 AI037692/AI/NIAID NIH HHS/United States
- 5U19DK080652-02/DK/NIDDK NIH HHS/United States
- U19 DK080652/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

