Surface contact stimulates the just-in-time deployment of bacterial adhesins
- PMID: 22053824
- PMCID: PMC3245333
- DOI: 10.1111/j.1365-2958.2011.07909.x
Surface contact stimulates the just-in-time deployment of bacterial adhesins
Abstract
The attachment of bacteria to surfaces provides advantages such as increasing nutrient access and resistance to environmental stress. Attachment begins with a reversible phase, often mediated by surface structures such as flagella and pili, followed by a transition to irreversible attachment, typically mediated by polysaccharides. Here we show that the interplay between pili and flagellum rotation stimulates the rapid transition between reversible and polysaccharide-mediated irreversible attachment. We found that reversible attachment of Caulobacter crescentus cells is mediated by motile cells bearing pili and that their contact with a surface results in the rapid pili-dependent arrest of flagellum rotation and concurrent stimulation of polar holdfast adhesive polysaccharide. Similar stimulation of polar adhesin production by surface contact occurs in Asticcacaulis biprosthecum and Agrobacterium tumefaciens. Therefore, single bacterial cells respond to their initial contact with surfaces by triggering just-in-time adhesin production. This mechanism restricts stable attachment to intimate surface interactions, thereby maximizing surface attachment, discouraging non-productive self-adherence, and preventing curing of the adhesive.
© 2011 Blackwell Publishing Ltd.
Figures



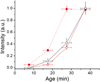

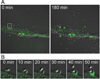
Comment in
-
Reflections on a sticky situation: how surface contact pulls the trigger for bacterial adhesion.Mol Microbiol. 2012 Jan;83(1):7-9. doi: 10.1111/j.1365-2958.2011.07913.x. Epub 2011 Nov 18. Mol Microbiol. 2012. PMID: 22092444
References
-
- Blair KM, Turner L, Winkelman JT, Berg HC, Kearns DB. A molecular clutch disables flagella in the Bacillus subtilis biofilm. Science. 2008;320:1636–1638. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

