Pleiotropic effects of pitavastatin
- PMID: 22053916
- PMCID: PMC3376429
- DOI: 10.1111/j.1365-2125.2011.04139.x
Pleiotropic effects of pitavastatin
Abstract
3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) are established first line treatments for hypercholesterolaemia. In addition to the direct effects of statins in reducing concentrations of atherogenic low density lipoprotein cholesterol (LDL-C), several studies have indicated that the beneficial effects of statins may be due to some of their cholesterol-independent, multiple (pleiotropic) effects which may differ between different members of the class. Pitavastatin is a novel synthetic lipophilic statin that has a number of pharmacodynamic and pharmacokinetic properties distinct from those of other statins, which may underlie its potential pleiotropic benefits in reducing cardiovascular risk factors. This review examines the principal pleiotropic effects of pitavastatin on endothelial function, vascular inflammation, oxidative stress and thrombosis. The article is based on a systematic literature search carried out in December 2010, together with more recent relevant publications where appropriate. The available data from clinical trials and in vitro and animal studies suggest that pitavastatin is not only effective in reducing LDL-C and triglycerides, but also has a range of other effects. These include increasing high density lipoprotein cholesterol, decreasing markers of platelet activation, improving cardiac, renal and endothelial function, and reducing endothelial stress, lipoprotein oxidation and, ultimately, improving the signs and symptoms of atherosclerosis. It is concluded that the diverse pleiotropic actions of pitavastatin may contribute to reducing cardiovascular morbidity and mortality beyond that achieved through LDL-C reduction.
© 2011 The Author. British Journal of Clinical Pharmacology © 2011 The British Pharmacological Society.
Figures

 )
)
 )
)
 )
)
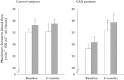
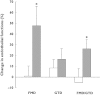
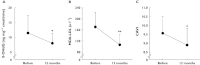
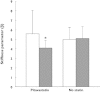
 ). 8-OHdG,8-hydroxy-2′-deoxyguanosine; LDL-C, low density lipoprotein cholesterol; L-FABP, L-fatty acid binding protein
). 8-OHdG,8-hydroxy-2′-deoxyguanosine; LDL-C, low density lipoprotein cholesterol; L-FABP, L-fatty acid binding protein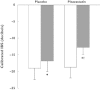
 )
)
References
-
- Dembowski E, Davidson MH. A review of lipid management in primary and secondary prevention. J Cardiopulm Rehabil Prev. 2009;29:2–12. - PubMed
-
- Sadowitz B, Maier KG, Gahtan V. Basic science review: statin therapy – Part I: the pleiotropic effects of statins in cardiovascular disease. Vasc Endovascular Surg. 2010;44:241–51. - PubMed
-
- Sadowitz B, Seymour K, Costanza MJ, Gahtan V. Basic science review section: statin therapy – Part II: clinical considerations for cardiovascular disease. Vasc Endovascular Surg. 2010;44:421–33. - PubMed
-
- Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors. Arterioscler Thromb Vasc Biol. 2001;21:1712–9. - PubMed
-
- Al-Hoqail IA. Personalized medicine in psoriasis: concept and applications. Curr Vasc Pharmacol. 2010;8:432–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

