Loss of interferon regulatory factor 5 (IRF5) expression in human ductal carcinoma correlates with disease stage and contributes to metastasis
- PMID: 22053985
- PMCID: PMC3326553
- DOI: 10.1186/bcr3053
Loss of interferon regulatory factor 5 (IRF5) expression in human ductal carcinoma correlates with disease stage and contributes to metastasis
Abstract
Introduction: New signaling pathways of the interleukin (IL) family, interferons (IFN) and interferon regulatory factors (IRF) have recently been found within tumor microenvironments and in metastatic sites. Some of these cytokines stimulate while others inhibit breast cancer proliferation and/or invasion. IRFs, a family of nine mammalian transcription factors, have multiple biologic functions that when dysregulated may contribute to tumorigenesis; most well-known are their roles in regulating/initiating host immunity. Some IRF family members have been implicated in tumorigenesis yet little is still known of their expression in primary human tumors or their role(s) in disease development/progression. IRF5 is one of the newer family members to be studied and has been shown to be a critical mediator of host immunity and the cellular response to DNA damage. Here, we examined the expression of IRF5 in primary breast tissue and determined how loss of expression may contribute to breast cancer development and/or progression.
Methods: Formalin-fixed paraffin-embedded archival breast tissue specimens from patients with atypical ductal hyperplasia (ADH), ductal carcinoma in situ (DCIS) and invasive ductal carcinoma (IDC) were examined for their expression of IRF1 and IRF5. Knockdown or overexpression of IRF5 in MCF-10A, MCF-7 and MDA-MB-231 mammary epithelial cell lines was used to examine the role of IRF5 in growth inhibition, invasion and tumorigenesis.
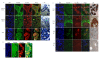
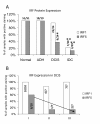
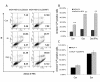
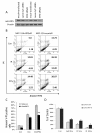
Results: Analysis of IRF expression in human breast tissues revealed the unique down-regulation of IRF5 in patients with different grades of DCIS and IDC as compared to IRF1; loss of IRF5 preceded that of IRF1 and correlated with increased invasiveness. Overexpression of IRF5 in breast cancer cells inhibited in vitro and in vivo cell growth and sensitized them to DNA damage. Complementary experiments with IRF5 siRNAs made normal mammary epithelial cells resistant to DNA damage. By 3-D culture, IRF5 overexpression reverted MDA-MB-231 to normal acini-like structures; cells overexpressing IRF5 had decreased CXCR4 expression and were insensitive to SDF-1/CXCL12-induced migration. These findings were confirmed by CXCR4 promoter reporter assays.
Conclusions: IRF5 is an important tumor suppressor that regulates multiple cellular processes involved in the conversion of normal mammary epithelial cells to tumor epithelial cells with metastatic potential.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

