Modern fluorescent proteins: from chromophore formation to novel intracellular applications
- PMID: 22054544
- PMCID: PMC4437206
- DOI: 10.2144/000113765
Modern fluorescent proteins: from chromophore formation to novel intracellular applications
Abstract
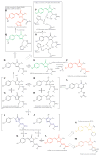
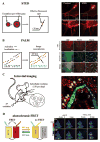
The diverse biochemical and photophysical properties of fluorescent proteins (FPs) have enabled the generation of a growing palette of colors, providing unique opportunities for their use in a variety of modern biology applications. Modulation of these FP characteristics is achieved through diversity in both the structure of the chromophore as well as the contacts between the chromophore and the surrounding protein barrel. Here we review our current knowledge of blue, green, and red chromophore formation in permanently emitting FPs, photoactivatable FPs, and fluorescent timers. Progress in understanding the interplay between FP structure and function has allowed the engineering of FPs with many desirable features, and enabled recent advances in microscopy techniques such as super-resolution imaging of single molecules, imaging of protein dynamics, photochromic FRET, deep-tissue imaging, and multicolor two-photon microscopy in live animals.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
