Phase II trial of adjuvant pelvic radiation "sandwiched" between ifosfamide or ifosfamide plus cisplatin in women with uterine carcinosarcoma
- PMID: 22055846
- PMCID: PMC3787514
- DOI: 10.1016/j.ygyno.2011.10.008
Phase II trial of adjuvant pelvic radiation "sandwiched" between ifosfamide or ifosfamide plus cisplatin in women with uterine carcinosarcoma
Abstract
Objective: Uterine carcinosarcoma (CS) is a rare uterine tumor with an extremely poor prognosis. In the adjuvant setting, efficacy has been shown with radiotherapy (RT), systemic chemotherapy, or both. This is the first report describing the efficacy and toxicity of adjuvant ifosfamide or ifosfamide plus cisplatin "sandwiched" with RT in patients with surgically staged and completely resected uterine carcinosarcoma.
Methods: Women with surgically staged CS with no gross residual disease were initially administered ifosfamide (1.2 g/m(2)/day×5 days) with cisplatin (20 mg/m(2)/day×5 days) every 3 weeks for 3 cycles followed by pelvic external beam RT and brachytherapy followed by 3 additional cycles of ifosfamide (1.0 g/m2/day) with cisplatin (20 mg/m(2)/day×5 days) every 3 weeks. Similar to the GOG trial in recurrent CS (Sutton et al., 2000), the addition of cisplatin added toxicity without additional efficacy, so mid-study, the cisplatin was eliminated from the regimen. Toxicities were recorded and disease-free survival (DFS) was calculated with Kaplan-Meier statistical methods.
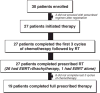
Results: In total, 12 patients received ifosfamide and cisplatin and 15 patients received ifosfamide alone, both 'sandwiched' with RT. The median follow up was 35.9 months (range 6-88). The 2 year DFS was similar in both the ifosfamide/cisplatin and ifosfamide groups (log-rank p=0.16), so they were combined for analysis. 19 patients (70%) completed the protocol. As expected, stage 1 patients had a better 2-year DFS (18.75 ± 1.12 months; log-rank p=0.008 when compared to stages 2, 3, 4). Also, in stages 2, 3 and 4 patients, the DFS was 15.81 ± 1.73 months. Grade 3/4 neutropenia, anemia and thrombocytopenia occurred in 18%, 4% and 4% of cycles, respectively.
Conclusions: Ifosfamide "sandwiched" with RT appears to be an efficacious regimen for surgically staged CS patients with no residual disease, even in patients with advanced stage. The addition of cisplatin to the regimen added toxicity without improving efficacy. Even with ifosfamide alone, the efficacy of this 'sandwich' regimen comes with a moderate but tolerable toxicity profile.
Trial registration: ClinicalTrials.gov NCT00231842.
Copyright © 2011. Published by Elsevier Inc.
Conflict of interest statement
The authors declare that there are no conflicts of interest
Figures

Comment in
-
[Adjuvant radiochemotherapy of uterine carcinosarcoma in need of optimization].Strahlenther Onkol. 2012 Sep;188(9):843-6. doi: 10.1007/s00066-012-0166-4. Strahlenther Onkol. 2012. PMID: 22915184 German. No abstract available.
Similar articles
-
A gynecologic oncology group randomized phase III trial of whole abdominal irradiation (WAI) vs. cisplatin-ifosfamide and mesna (CIM) as post-surgical therapy in stage I-IV carcinosarcoma (CS) of the uterus.Gynecol Oncol. 2007 Nov;107(2):177-85. doi: 10.1016/j.ygyno.2007.07.070. Epub 2007 Sep 5. Gynecol Oncol. 2007. PMID: 17822748 Free PMC article. Clinical Trial.
-
A retrospective assessment of outcomes of chemotherapy-based versus radiation-only adjuvant treatment for completely resected stage I-IV uterine carcinosarcoma.Gynecol Oncol. 2008 Nov;111(2):249-54. doi: 10.1016/j.ygyno.2008.06.035. Epub 2008 Aug 27. Gynecol Oncol. 2008. PMID: 18755503 Free PMC article.
-
Adjuvant ifosfamide and cisplatin in patients with completely resected stage I or II carcinosarcomas (mixed mesodermal tumors) of the uterus: a Gynecologic Oncology Group study.Gynecol Oncol. 2005 Mar;96(3):630-4. doi: 10.1016/j.ygyno.2004.11.022. Gynecol Oncol. 2005. PMID: 15721404 Clinical Trial.
-
[Uterine carcinosarcoma].Ginekol Pol. 2012 Aug;83(8):609-12. Ginekol Pol. 2012. PMID: 23342885 Review. Polish.
-
The role of ifosfamide and systemic therapy in the management of carcinoma of the cervix.Semin Oncol. 1996 Jun;23(3 Suppl 6):56-64. Semin Oncol. 1996. PMID: 8677451 Review.
Cited by
-
Uterine carcinosarcoma: A 10-year single institution experience.PLoS One. 2022 Jul 21;17(7):e0271526. doi: 10.1371/journal.pone.0271526. eCollection 2022. PLoS One. 2022. PMID: 35862371 Free PMC article.
-
A multi-institutional study of outcomes in stage I-III uterine carcinosarcoma.Gynecol Oncol. 2015 Nov;139(2):275-82. doi: 10.1016/j.ygyno.2015.09.002. Epub 2015 Sep 6. Gynecol Oncol. 2015. PMID: 26348313 Free PMC article.
-
Adjuvant chemo-radiotherapy in the "sandwich" method for high risk endometrial cancer--a review of literature.BMC Womens Health. 2015 Jun 24;15:50. doi: 10.1186/s12905-015-0207-0. BMC Womens Health. 2015. PMID: 26104468 Free PMC article.
-
[Adjuvant radiochemotherapy of uterine carcinosarcoma in need of optimization].Strahlenther Onkol. 2012 Sep;188(9):843-6. doi: 10.1007/s00066-012-0166-4. Strahlenther Onkol. 2012. PMID: 22915184 German. No abstract available.
-
Surgical outcomes for 131 cases of carcinosarcoma of the hepatobiliary tract.J Gastroenterol. 2014 Jun;49(6):982-91. doi: 10.1007/s00535-013-0882-2. Epub 2013 Oct 26. J Gastroenterol. 2014. PMID: 24162331 Review.
References
-
- Sherman ME, Devesa SS. Analysis of racial differences in incidence, survival, and mortality for malignant tumors of the uterine corpus. Cancer. 2003 Jul 1;98(1):176–86. - PubMed
-
- Nordal RR, Thoresen SO. Uterine sarcomas in Norway 1956–1992: incidence, survival and mortality. Eur J Cancer. 1997 May;33(6):907–11. - PubMed
-
- Major FJ, Blessing JA, Silverberg SG, Morrow CP, Creasman WT, Currie JL, et al. Prognostic factors in early-stage uterine sarcoma. A Gynecologic Oncology Group study. Cancer. 1993 Feb 15;71(4 Suppl):1702–9. - PubMed
-
- Rose PG, Piver MS, Tsukada Y, Lau T. Patterns of metastasis in uterine sarcoma. An autopsy study. Cancer. 1989 Mar 1;63(5):935–8. - PubMed
-
- Chen SS. Propensity of retroperitoneal lymph node metastasis in patients with stage I sarcoma of the uterus. Gynecol Oncol. 1989 Feb;32(2):215–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

