Epac enhances excitation-transcription coupling in cardiac myocytes
- PMID: 22056318
- PMCID: PMC3246099
- DOI: 10.1016/j.yjmcc.2011.10.016
Epac enhances excitation-transcription coupling in cardiac myocytes
Abstract
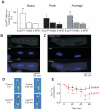
Epac is a guanine nucleotide exchange protein that is directly activated by cAMP, but whose cardiac cellular functions remain unclear. It is important to understand cardiac Epac signaling, because it is activated in parallel to classical cAMP-dependent signaling via protein kinase A. In addition to activating contraction, Ca(2+) is a key cardiac transcription regulator (excitation-transcription coupling). It is unknown how myocyte Ca(2+) signals are decoded in cardiac myocytes to control nuclear transcription. We examine Epac actions on cytosolic ([Ca(2+)](i)) and intranuclear ([Ca(2+)](n)) Ca(2+) homeostasis, focusing on whether Epac alters [Ca(2+)](n) and activates a prohypertrophic program in cardiomyocytes. Adult rat cardiomyocytes, loaded with fluo-3 were viewed by confocal microscopy during electrical field stimulation at 1Hz. Acute Epac activation by 8-pCPT increased Ca(2+) sparks and diastolic [Ca(2+)](i), but decreased systolic [Ca(2+)](i). The effects on diastolic [Ca(2+)](i) and Ca(2+) spark frequency were dependent on phospholipase C (PLC), inositol 1,4,5 triphosphate receptor (IP(3)R) and CaMKII activation. Interestingly, Epac preferentially increased [Ca(2+)](n) during both diastole and systole, correlating with the perinuclear expression pattern of Epac. Moreover, Epac activation induced histone deacetylase 5 (HDAC5) nuclear export, with consequent activation of the prohypertrophic transcription factor MEF2. These data provide the first evidence that the cAMP-binding protein Epac modulates cardiac nuclear Ca(2+) signaling by increasing [Ca(2+)](n) through PLC, IP(3)R and CaMKII activation, and initiates a prohypertrophic program via HDAC5 nuclear export and subsequent activation of the transcription factor MEF2.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures





References
-
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. - PubMed
-
- Morel E, Marcantoni A, Gastineau M, Birkedal R, Rochais F, Garnier A, et al. cAMP-binding protein Epac induces cardiomyocyte hypertrophy. Circ Res. 2005;97:1296–304. - PubMed
-
- Metrich M, Lucas A, Gastineau M, Samuel JL, Heymes C, Morel E, et al. Epac mediates beta-adrenergic receptor-induced cardiomyocyte hypertrophy. Circ Res. 2008;102:959–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

