Connexins protect mouse pancreatic β cells against apoptosis
- PMID: 22056383
- PMCID: PMC3225984
- DOI: 10.1172/JCI40509
Connexins protect mouse pancreatic β cells against apoptosis
Abstract
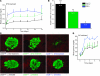
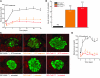
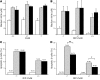
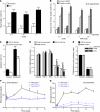
Type 1 diabetes develops when most insulin-producing β cells of the pancreas are killed by an autoimmune attack. The in vivo conditions modulating the sensitivity and resistance of β cells to this attack remain largely obscure. Here, we show that connexin 36 (Cx36), a trans-membrane protein that forms gap junctions between β cells in the pancreatic islets, protects mouse β cells against both cytotoxic drugs and cytokines that prevail in the islet environment at the onset of type 1 diabetes. We documented that this protection was at least partially dependent on intercellular communication, which Cx36 and other types of connexin channels establish within pancreatic islets. We further found that proinflammatory cytokines decreased expression of Cx36 and that experimental reduction or augmentation of Cx36 levels increased or decreased β cell apoptosis, respectively. Thus, we conclude that Cx36 is central to β cell protection from toxic insults.
Figures

References
-
- Bavamian S, et al. Islet-cell-to-cell communication as basis for normal insulin secretion. Diabetes Obes Metab. 2007;9 suppl 2:118–132. - PubMed
-
- Theis M, et al. Replacement by a lacZ reporter gene assigns mouse connexin36, 45 and 43 to distinct cell types in pancreatic islets. Exp Cell Res. 2004;294(1):18–29. - PubMed
-
- Harris AL, Locke D, eds.Connexin: A Guide . New York, New York, USA: Springer; 2009.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

