The cost-effectiveness of birth-cohort screening for hepatitis C antibody in U.S. primary care settings
- PMID: 22056542
- PMCID: PMC5484577
- DOI: 10.7326/0003-4819-156-4-201202210-00378
The cost-effectiveness of birth-cohort screening for hepatitis C antibody in U.S. primary care settings
Abstract
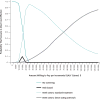
Background: In the United States, hepatitis C virus (HCV) infection is most prevalent among adults born from 1945 through 1965, and approximately 50% to 75% of infected adults are unaware of their infection.
Objective: To estimate the cost-effectiveness of birth-cohort screening.
Design: Cost-effectiveness simulation.
Data sources: National Health and Nutrition Examination Survey, U.S. Census, Medicare reimbursement schedule, and published sources.
Target population: Adults born from 1945 through 1965 with 1 or more visits to a primary care provider annually.
Time horizon: Lifetime.
Perspective: Societal, health care.
Intervention: One-time antibody test of 1945-1965 birth cohort.
Outcome measures: Numbers of cases that were identified and treated and that achieved a sustained viral response; liver disease and death from HCV; medical and productivity costs; quality-adjusted life-years (QALYs); incremental cost-effectiveness ratio (ICER).
Results of base-case analysis: Compared with the status quo, birth-cohort screening identified 808,580 additional cases of chronic HCV infection at a screening cost of $2874 per case identified. Assuming that birth-cohort screening was followed by pegylated interferon and ribavirin (PEG-IFN+R) for treated patients, screening increased QALYs by 348,800 and costs by $5.5 billion, for an ICER of $15,700 per QALY gained. Assuming that birth-cohort screening was followed by direct-acting antiviral plus PEG-IFN+R treatment for treated patients, screening increased QALYs by 532,200 and costs by $19.0 billion, for an ICER of $35,700 per QALY saved.
Results of sensitivity analysis: The ICER of birth-cohort screening was most sensitive to sustained viral response of antiviral therapy, the cost of therapy, the discount rate, and the QALY losses assigned to disease states.
Limitation: Empirical data on screening and direct-acting antiviral treatment in real-world clinical settings are scarce.
Conclusion: Birth-cohort screening for HCV in primary care settings was cost-effective.
Primary funding source: Division of Viral Hepatitis, Centers for Disease Control and Prevention.
Conflict of interest statement
Figures

Comment in
-
Hepatitis C: the end of the beginning and possibly the beginning of the end.Ann Intern Med. 2012 Feb 21;156(4):317-8. doi: 10.7326/0003-4819-156-4-201202210-00014. Ann Intern Med. 2012. PMID: 22351718 Free PMC article. No abstract available.
-
A great time to invest in baby Boomer's hepatitis C!Hepatology. 2012 Oct;56(4):1575-7. doi: 10.1002/hep.25898. Hepatology. 2012. PMID: 23038650 No abstract available.
-
The cost-effectiveness of birth cohort screening for hepatitis C antibody in US primary care settings.Gastroenterology. 2013 Feb;144(2):457-459. doi: 10.1053/j.gastro.2012.12.013. Epub 2012 Dec 20. Gastroenterology. 2013. PMID: 23260498 No abstract available.
References
-
- Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–14. - PubMed
-
- Thomas DL, Seeff LB. Natural history of hepatitis C. Clin Liver Dis. 2005;9:383–98. - PubMed
-
- Davis GL, Albright JE, Cook SF, Rosenberg DM. Projecting future complications of chronic hepatitis C in the United States. Liver Transpl. 2003;9:331–8. - PubMed
-
- Deuffic-Burban S, Poynard T, Sulkowski MS, Wong JB. Estimating the future health burden of chronic hepatitis C and human immunodeficiency virus infections in the United States. J Viral Hepat. 2007;14:107–15. - PubMed
-
- Rein DB, Wittenborn JS, Weinbaum CM, Sabin M, Smith BD, Lesesne SB. Forecasting the morbidity and mortality associated with prevalent cases of pre-cirrhotic chronic hepatitis C in the United States. Dig Liver Dis. 2011;43:66–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
