Cdk1 promotes kinetochore bi-orientation and regulates Cdc20 expression during recovery from spindle checkpoint arrest
- PMID: 22056777
- PMCID: PMC3261552
- DOI: 10.1038/emboj.2011.385
Cdk1 promotes kinetochore bi-orientation and regulates Cdc20 expression during recovery from spindle checkpoint arrest
Abstract
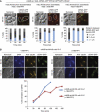
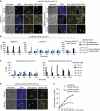
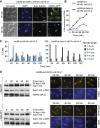
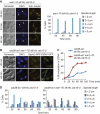
The spindle assembly checkpoint (SAC), an evolutionarily conserved surveillance pathway, prevents chromosome segregation in response to conditions that disrupt the kinetochore-microtubule attachment. Removal of the checkpoint-activating stimulus initiates recovery during which spindle integrity is restored, kinetochores become bi-oriented, and cells initiate anaphase. Whether recovery ensues passively after the removal of checkpoint stimulus, or requires mediation by specific effectors remains uncertain. Here, we report two unrecognized functions of yeast Cdk1 required for efficient recovery from SAC-induced arrest. We show that Cdk1 promotes kinetochore bi-orientation during recovery by restraining premature spindle elongation thereby extinguishing SAC signalling. Moreover, Cdk1 is essential for sustaining the expression of Cdc20, an activator of the anaphase promoting complex/cyclosome (APC/C) required for anaphase progression. We suggest a model in which Cdk1 activity promotes recovery from SAC-induced mitotic arrest by regulating bi-orientation and APC/C activity. Our findings provide fresh insights into the regulation of mitosis and have implications for the therapeutic efficacy of anti-mitotic drugs.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Amon A (1999) The spindle checkpoint. Curr Opin Genet Dev 9: 69–75 - PubMed
-
- Bishop AC, Ubersax JA, Petsch DT, Matheos DP, Gray NS, Blethrow J, Shimizu E, Tsien JZ, Schultz PG, Rose MD, Wood JL, Morgan DO, Shokat KM (2000) A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature 407: 395–401 - PubMed
-
- Cross F, Tinkelenberg AH (1991) A positive feedback loop controlling CLN1 and CLN2 gene expression at the START of yeast cell cycle. Cell 65: 875–883 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

