SOHLH1 and SOHLH2 coordinate spermatogonial differentiation
- PMID: 22056784
- PMCID: PMC3249242
- DOI: 10.1016/j.ydbio.2011.10.027
SOHLH1 and SOHLH2 coordinate spermatogonial differentiation
Abstract
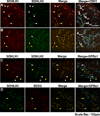
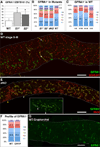
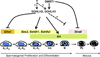
Spermatogonial self-renewal and differentiation are essential for male fertility and reproduction. We discovered that germ cell specific genes Sohlh1 and Sohlh2, encode basic helix-loop-helix (bHLH) transcriptional regulators that are essential in spermatogonial differentiation. Sohlh1 and Sohlh2 individual mouse knockouts show remarkably similar phenotypes. Here we show that SOHLH1 and SOHLH2 proteins are co-expressed in the entire spermatogonial population except in the GFRA1(+) spermatogonia, which includes spermatogonial stem cells (SSCs). SOHLH1 and SOHLH2 are expressed in both KIT negative and KIT positive spermatogonia, and overlap Ngn3/EGFP and SOX3 expression. SOHLH1 and SOHLH2 heterodimerize with each other in vivo, as well as homodimerize. The Sohlh1/Sohlh2 double mutant phenocopies single mutants, i.e., spermatogonia continue to proliferate but do not differentiate properly. Further analysis revealed that GFRA1(+) population was increased, while meiosis commenced prematurely in both single and double knockouts. Sohlh1 and Sohlh2 double deficiency has a synergistic effect on gene expression patterns as compared to the single knockouts. SOHLH proteins affect spermatogonial development by directly regulating Gfra1, Sox3 and Kit gene expression. SOHLH1 and SOHLH2 suppress genes involved in SSC maintenance, and induce genes important for spermatogonial differentiation.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures





References
-
- Baleato RM, Aitken RJ, Roman SD. Vitamin A regulation of BMP4 expression in the male germ line. Dev Biol. 2005;286:78–90. - PubMed
-
- Ballow D, Meistrich ML, Matzuk M, Rajkovic A. Sohlh1 is essential for spermatogonial differentiation. Dev Biol. 2006a;294:161–167. - PubMed
-
- Ballow DJ, Xin Y, Choi Y, Pangas SA, Rajkovic A. Sohlh2 is a germ cell-specific bHLH transcription factor. Gene Expr Patterns. 2006b;6:1014–1018. - PubMed
-
- Baltus AE, Menke DB, Hu YC, Goodheart ML, Carpenter AE, de Rooij DG, Page DC. In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat Genet. 2006;38:1430–1434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

