A novel melano-lysosome in the retinal epithelium of rhesus monkeys
- PMID: 22056912
- PMCID: PMC6314486
- DOI: 10.1016/j.exer.2011.10.011
A novel melano-lysosome in the retinal epithelium of rhesus monkeys
Abstract
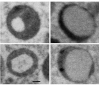
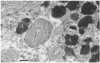
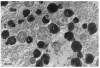
The large phagocytic load that confronts the retinal pigment epithelium (RPE) is thought to play a possible role in the pathogenesis of age related macular degeneration (AMD) that afflicts both humans and monkeys. Our knowledge of how RPE degrades phagosomes and other intra-cellular material by lysosomal action is still rudimentary. In this paper we examine organelles that play a role in this process, melanosome, lysosomes and phagosomes, in the RPE of young and old rhesus monkeys in order to better understand lysosomal autophagy and heterophagy in the RPE and its possible role in AMD. We used electron microscopy to detect and describe the characteristics of melanosomes and lysosome-like organelles in the macular RPE of rhesus monkeys (Macaca mulatta) that were 1, 6, 24, 24, 26 and 35 years of age. The measurements include the number, shape and size of these organelles located in the basal, middle and apical regions of RPE cells. Phaagosomes were also examined but not counted or measured for size or shape because of their rarity. Melanosomes were homogeneously dark with a circular or elliptical shape and decreased in number with age. Smaller melanosomes were more common at the basal side of the RPE. Among the small melanosomes, we found an organelle that was losing melanin in varying degrees; in some cases was nearly devoid of melanin. Because of the melanin loss, we considered this organelle to be a unique type of autophagic melano-lysosome, which we called a Type 1 lysosome. We found another organelle, more canonically lysosomal, which we called a Type 2 lysosome. This organelle was composed of a light matrix containing melanosomes in various stages of degradation. Type 2 lysosomes without melanosomes were rare. Type 2 lysosomes increased while Type 1 decreased in number with age. Phagosomes were rare in both young and old monkeys. They made close contact with Type 2 lysosomes which we considered responsible for their degradation. Melanosomes are being lost from monkey RPE with age. Much of this loss is carried out by two types of lysosomes. One, not defined as unique before, appears to be autophagic in digesting its own melanin; it has been called a Type 1 lysosome. The other, a more canonical lysosome, is both heterophagic in digesting phagosomes and autophagic in digesting local melanosomes; it has been called a Type 2 lysosome. Type 1 lysosomes decrease while type 2 lysosomes increase with age. The loss of melanin is considered to be detrimental to the RPE since it reduces melanin's protective action against light toxicity and oxidative stress. Phagosomes appear to be degraded by membrane contacts with Type 2 lysosomes. The loss of melanin and the buildup of Type 2 lysosomes occur at an earlier age in monkeys than humans implying that a greater vulnerability to senescence accelerates the rate of AMD in monkeys.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Conflict of interest statement
None to declare.
Figures














References
-
- Bergmann M, Schutt F, Holz FG, Kopitz J. Inhibition of the ATP driven proton pump in RPE lysosomes by the major lipofuscin fluorophore A2-E may contribute to the pathogenesis of age-related macular degeneration. FASEB J. 2004;18:562–564. - PubMed
-
- Bosch E, Horwitz J, Bok D. Phagocytosis of outer segments by retinal pigment epithelium: phagosome–lysosome interaction. J Histochem Cytochem. 1993;41:253–263. - PubMed
-
- Brandes D, Anton E. Lysosomes in uterine involution: intracytoplasmic degradation of myofilaments and collagen. J Gerontol. 1969;24:55–69. - PubMed
-
- Burke JM, Skumatz MB. Autofluorescent inclusions in long term postconfluent cultures of retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1998;39:1478–1486. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

