Chromatin-associated RNA interference components contribute to transcriptional regulation in Drosophila
- PMID: 22056986
- PMCID: PMC4082306
- DOI: 10.1038/nature10492
Chromatin-associated RNA interference components contribute to transcriptional regulation in Drosophila
Abstract
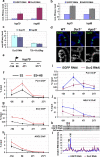
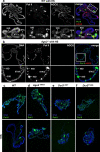
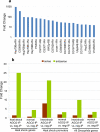
RNA interference (RNAi) pathways have evolved as important modulators of gene expression that operate in the cytoplasm by degrading RNA target molecules through the activity of short (21-30 nucleotide) RNAs. RNAi components have been reported to have a role in the nucleus, as they are involved in epigenetic regulation and heterochromatin formation. However, although RNAi-mediated post-transcriptional gene silencing is well documented, the mechanisms of RNAi-mediated transcriptional gene silencing and, in particular, the role of RNAi components in chromatin dynamics, especially in animal multicellular organisms, are elusive. Here we show that the key RNAi components Dicer 2 (DCR2) and Argonaute 2 (AGO2) associate with chromatin (with a strong preference for euchromatic, transcriptionally active, loci) and interact with the core transcription machinery. Notably, loss of function of DCR2 or AGO2 showed that transcriptional defects are accompanied by the perturbation of RNA polymerase II positioning on promoters. Furthermore, after heat shock, both Dcr2 and Ago2 null mutations, as well as missense mutations that compromise the RNAi activity, impaired the global dynamics of RNA polymerase II. Finally, the deep sequencing of the AGO2-associated small RNAs (AGO2 RIP-seq) revealed that AGO2 is strongly enriched in small RNAs that encompass the promoter regions and other regions of heat-shock and other genetic loci on both the sense and antisense DNA strands, but with a strong bias for the antisense strand, particularly after heat shock. Taken together, our results show that DCR2 and AGO2 are globally associated with transcriptionally active loci and may have a pivotal role in shaping the transcriptome by controlling the processivity of RNA polymerase II.
Figures

Comment in
-
Gene regulation: RNAi gets stuck into transcription.Nat Rev Genet. 2011 Nov 22;13(1):2. doi: 10.1038/nrg3136. Nat Rev Genet. 2011. PMID: 22105085 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

