Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease
- PMID: 22056989
- PMCID: PMC3245796
- DOI: 10.1038/nature10648
Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease
Abstract
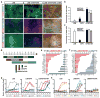
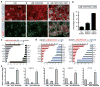
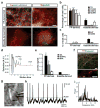
Human pluripotent stem cells (PSCs) are a promising source of cells for applications in regenerative medicine. Directed differentiation of PSCs into specialized cells such as spinal motoneurons or midbrain dopamine (DA) neurons has been achieved. However, the effective use of PSCs for cell therapy has lagged behind. Whereas mouse PSC-derived DA neurons have shown efficacy in models of Parkinson's disease, DA neurons from human PSCs generally show poor in vivo performance. There are also considerable safety concerns for PSCs related to their potential for teratoma formation or neural overgrowth. Here we present a novel floor-plate-based strategy for the derivation of human DA neurons that efficiently engraft in vivo, suggesting that past failures were due to incomplete specification rather than a specific vulnerability of the cells. Midbrain floor-plate precursors are derived from PSCs 11 days after exposure to small molecule activators of sonic hedgehog (SHH) and canonical WNT signalling. Engraftable midbrain DA neurons are obtained by day 25 and can be maintained in vitro for several months. Extensive molecular profiling, biochemical and electrophysiological data define developmental progression and confirm identity of PSC-derived midbrain DA neurons. In vivo survival and function is demonstrated in Parkinson's disease models using three host species. Long-term engraftment in 6-hydroxy-dopamine-lesioned mice and rats demonstrates robust survival of midbrain DA neurons derived from human embryonic stem (ES) cells, complete restoration of amphetamine-induced rotation behaviour and improvements in tests of forelimb use and akinesia. Finally, scalability is demonstrated by transplantation into parkinsonian monkeys. Excellent DA neuron survival, function and lack of neural overgrowth in the three animal models indicate promise for the development of cell-based therapies in Parkinson's disease.
Figures

Comment in
-
Neural development: From floorplate to function.Nat Rev Neurosci. 2011 Nov 30;13(1):2. doi: 10.1038/nrn3157. Nat Rev Neurosci. 2011. PMID: 22127302 No abstract available.
-
Stem cells: from animal models to clinical practice.J Neurol. 2012 Jun;259(6):1257-9. doi: 10.1007/s00415-012-6555-x. J Neurol. 2012. PMID: 22619060 No abstract available.
-
Improved viability of stem cell transplants in animal models of Parkinson's disease.Mov Disord. 2012 Jun;27(7):814. doi: 10.1002/mds.25036. Mov Disord. 2012. PMID: 22912974 No abstract available.
References
-
- Li XJ, et al. Specification of motoneurons from human embryonic stem cells. Nat Biotechnol. 2005;23:215–221. - PubMed
Publication types
MeSH terms
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

