The Src family kinase Fyn mediates hyperosmolarity-induced Mrp2 and Bsep retrieval from canalicular membrane
- PMID: 22057277
- PMCID: PMC3247936
- DOI: 10.1074/jbc.M111.292896
The Src family kinase Fyn mediates hyperosmolarity-induced Mrp2 and Bsep retrieval from canalicular membrane
Abstract
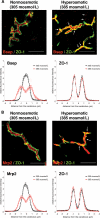
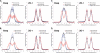
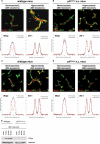
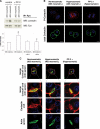
In perfused rat liver, hyperosmolarity induces Mrp2- (Kubitz, R., D'urso, D., Keppler, D., and Häussinger, D. (1997) Gastroenterology 113, 1438-1442) and Bsep retrieval (Schmitt, M., Kubitz, R., Lizun, S., Wettstein, M., and Häussinger, D. (2001) Hepatology 33, 509-518) from the canalicular membrane leading to cholestasis. The aim of this study was to elucidate the underlying signaling events. Hyperosmolarity-induced retrieval of Mrp2 and Bsep from the canalicular membrane in perfused rat liver was accompanied by an activating phosphorylation of the Src kinases Fyn and Yes but not of c-Src. Both hyperosmotic transporter retrieval and Src kinase activation were sensitive to apocynin (300 μmol/liter), N-acetylcysteine (NAC; 10 mmol/liter), and SU6656 (1 μmol/liter). Also PP-2 (250 nmol/liter), which inhibited hyperosmotic Fyn but not Yes activation, prevented hyperosmotic transporter retrieval from the canalicular membrane, suggesting that Fyn but not Yes mediates hyperosmotic Bsep and Mrp2 retrieval. Neither hyperosmotic Fyn activation nor Bsep/Mrp2 retrieval was observed in livers from p47(phox) knock-out mice. Hyperosmotic activation of JNKs was sensitive to apocynin and NAC but insensitive to SU6656 and PP-2, indicating that JNKs are not involved in transporter retrieval, as also evidenced by experiments using the JNK inhibitors L-JNKI-1 and SP6001255, respectively. Hyperosmotic transporter retrieval was accompanied by a NAC and Fyn knockdown-sensitive inhibition of biliary excretion of the glutathione conjugate of 1-chloro-2,4-dinitrobenzene in perfused rat liver and of cholyl-L-lysyl-fluorescein secretion into the pseudocanaliculi formed by hepatocyte couplets. Hyperosmolarity triggered an association between Fyn and cortactin and increased the amount of phosphorylated cortactin underneath the canalicular membrane. It is concluded that the hyperosmotic cholestasis is triggered by a NADPH oxidase-driven reactive oxygen species formation that mediates Fyn-dependent retrieval of the Mrp2 and Bsep from the canalicular membrane, which may involve an increased cortactin phosphorylation.
Figures



References
-
- Keppler D., Konig J. (1997) FASEB J. 11, 509–516 - PubMed
-
- Gatmaitan Z. C., Arias I. M. (1995) Physiol. Rev. 75, 261–275 - PubMed
-
- Müller M., Jansen P. L. (1997) Am. J. Physiol. 272, 1285–1303 - PubMed
-
- Meier P. J. (1995) Am. J. Physiol. 268, 801–812 - PubMed
-
- Vos T. A., Hooiveld G. J., Koning H., Childs S., Meijer D. K., Moshage H., Jansen P. L., Müller M. (1998) Hepatology 28, 1637–1644 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

