Identification of a central role for complement in osteoarthritis
- PMID: 22057346
- PMCID: PMC3257059
- DOI: 10.1038/nm.2543
Identification of a central role for complement in osteoarthritis
Abstract
Osteoarthritis, characterized by the breakdown of articular cartilage in synovial joints, has long been viewed as the result of 'wear and tear'. Although low-grade inflammation is detected in osteoarthritis, its role is unclear. Here we identify a central role for the inflammatory complement system in the pathogenesis of osteoarthritis. Through proteomic and transcriptomic analyses of synovial fluids and membranes from individuals with osteoarthritis, we find that expression and activation of complement is abnormally high in human osteoarthritic joints. Using mice genetically deficient in complement component 5 (C5), C6 or the complement regulatory protein CD59a, we show that complement, specifically, the membrane attack complex (MAC)-mediated arm of complement, is crucial to the development of arthritis in three different mouse models of osteoarthritis. Pharmacological modulation of complement in wild-type mice confirmed the results obtained with genetically deficient mice. Expression of inflammatory and degradative molecules was lower in chondrocytes from destabilized joints from C5-deficient mice than C5-sufficient mice, and MAC induced production of these molecules in cultured chondrocytes. Further, MAC colocalized with matrix metalloprotease 13 (MMP13) and with activated extracellular signal-regulated kinase (ERK) around chondrocytes in human osteoarthritic cartilage. Our findings indicate that dysregulation of complement in synovial joints has a key role in the pathogenesis of osteoarthritis.
Figures
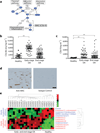
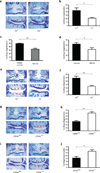
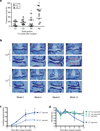
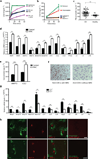
Comment in
-
Osteoarthritis: Complement-mediated inflammation in OA progression.Nat Rev Rheumatol. 2011 Dec 6;8(1):2. doi: 10.1038/nrrheum.2011.182. Nat Rev Rheumatol. 2011. PMID: 22143385 No abstract available.
References
-
- Felson DT. Clinical practice. Osteoarthritis of the knee. N Engl J Med. 2006;354:841–848. - PubMed
-
- Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213:626–634. - PubMed
-
- Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001;44:1237–1247. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

