Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules
- PMID: 22057348
- PMCID: PMC3233641
- DOI: 10.1038/nm.2554
Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules
Abstract
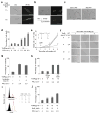
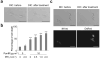
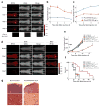
Three major modes of cancer therapy (surgery, radiation and chemotherapy) are the mainstay of modern oncologic therapy. To minimize the side effects of these therapies, molecular-targeted cancer therapies, including armed antibody therapy, have been developed with limited success. In this study, we have developed a new type of molecular-targeted cancer therapy, photoimmunotherapy (PIT), that uses a target-specific photosensitizer based on a near-infrared (NIR) phthalocyanine dye, IR700, conjugated to monoclonal antibodies (mAbs) targeting epidermal growth factor receptors. Cell death was induced immediately after irradiating mAb-IR700-bound target cells with NIR light. We observed in vivo tumor shrinkage after irradiation with NIR light in target cells expressing the epidermal growth factor receptor. The mAb-IR700 conjugates were most effective when bound to the cell membrane and produced no phototoxicity when not bound, suggesting a different mechanism for PIT as compared to conventional photodynamic therapies. Target-selective PIT enables treatment of cancer based on mAb binding to the cell membrane.
Figures


References
-
- Waldmann TA. Immunotherapy: past, present and future. Nat Med. 2003;9:269–277. - PubMed
-
- Reichert JM, Rosensweig CJ, Faden LB, Dewitz MC. Monoclonal antibody successes in the clinic. Nat Biotechnol. 2005;23:1073–1078. - PubMed
-
- Goldenberg DM, Sharkey RM, Paganelli G, Barbet J, Chatal JF. Antibody pretargeting advances cancer radioimmunodetection and radioimmunotherapy. J Clin Oncol. 2006;24:823–834. - PubMed
-
- Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ. Immunotoxin therapy of cancer. Nat Rev Cancer. 2006;6:559–565. - PubMed
-
- Mew D, Wat CK, Towers GH, Levy JG. Photoimmunotherapy: treatment of animal tumors with tumor-specific monoclonal antibody-hematoporphyrin conjugates. J Immunol. 1983;130:1473–1477. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

