General consideration on sialic acid chemistry
- PMID: 22057516
- PMCID: PMC11288308
- DOI: 10.1007/978-1-61779-373-8_3
General consideration on sialic acid chemistry
Abstract
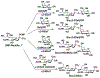
Sialic acids, also known as neuraminic acids, are a family of negatively charged α-keto acids with a nine-carbon backbone. These unique sugars have been found at the termini of many glycan chains of vertebrate cell surface, which play pivotal roles in mediating or modulating a variety of physiological and pathological processes. This brief review covers general approaches for synthesizing sialic acid containing structures. Recently developed synthetic methods along with structural diversities and biological functions of sialic acid are discussed.
Figures



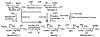
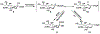
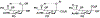

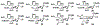
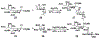
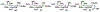
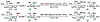
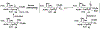

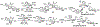
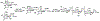
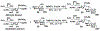
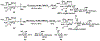
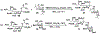
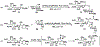





References
-
- Angata T, and Varki A (2002) Chemical diversity in the sialic acids and related alpha-keto acids: An evolutionary perspective. Chem. Rev 102, 439–469. - PubMed
-
- Kiefel MJ, and von Itzstein M (2002) Recent advances in the synthesis of sialic acid derivatives and sialylmimetics as biological probes. Chem. Rev 102, 471–490. - PubMed
-
- Bhattacharjee AK, Jennings HJ, Kenny CP, Martin A, and Smith IC (1975) Structural determination of the sialic acid polysaccharide antigens of Neisseria meningitidis serogroups B and C with carbon 13 nuclear magnetic resonance. J. Biol. Chem 250, 1926–1932. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

