Selective tau tyrosine nitration in non-AD tauopathies
- PMID: 22057784
- PMCID: PMC3314430
- DOI: 10.1007/s00401-011-0898-8
Selective tau tyrosine nitration in non-AD tauopathies
Abstract
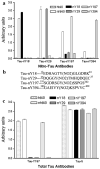
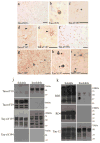
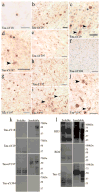
Previously, we reported the characterization of two novel antibodies that react with tau nitrated at tyrosine 197 (Tau-nY197) and tyrosine 394 (Tau-nY394) in Alzheimer's disease (AD). In this report, we examined whether tau nitration at these sites also occurs in corticobasal degeneration (CBD), progressive supranuclear palsy (PSP) and Pick's disease (PiD), three neurodegenerative tauopathies that contain abundant tau deposits within glial and neuronal cell types but lack amyloid deposition. The reactivity of these antibodies was also compared to two previously characterized antibodies Tau-nY18 and Tau-nY29, specific for tau nitrated at tyrosine 18 and tyrosine 29, respectively. In the present experiments, Tau-nY18 did not label the classical pathological lesions of CBD or PSP but did label the neuronal lesions associated with PiD to a limited extent. In contrast, Tau-nY29 revealed some, but not all classes of tau inclusions associated with both CBD and PSP but did label numerous Pick body inclusions in PiD. Tau-nY197 was restricted to the neuropil threads in both CBD and PSP; however, similar to Tau-nY29, extensive Pick body pathology was clearly labeled. Tau-nY394 did not detect any of the lesions associated with these disorders. In contrast, extensive neuronal and glial tau pathology within these diseases was labeled by Tau-Y197, a monoclonal antibody that reacts within the Y-197-containing proline-rich region of the molecule. Based on our Western and IHC experiments, it appears that nitration of tau at tyrosine 29 is a pathological modification that might be associated with neurodegeneration. Collectively, our data suggest that site-specific tau tyrosine nitration events occur in a disease and lesion-specific manner, indicating that nitration appears to be a highly controlled modification in AD and non-AD tauopathies.
Figures


References
-
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992;42(3 Pt 1):631–639. - PubMed
-
- Berry RW, Sweet AP, Clark FA, Lagalwar S, Lapin BR, Wang T, Topgi S, Guillozet-Bongaarts AL, Cochran EJ, Bigio EH, Binder LI. Tau epitope display in progressive supranuclear palsy and corticobasal degeneration. J Neurocytol. 2004;33(3):287–295. - PubMed
-
- Braak H, Braak E. Evolution of the neuropathology of Alzheimer’s disease. Acta Neurol Scand Suppl. 1996;165:3–12. - PubMed
-
- Carmel G, Mager EM, Binder LI, Kuret J. The structural basis of monoclonal antibody Alz50’s selectivity for Alzheimer’s disease pathology. J Biol Chem. 1996;271(51):32789–32795. - PubMed
-
- de Silva R, Lashley T, Strand C, Shiarli AM, Shi J, Tian J, Bailey KL, Davies P, Bigio EH, Arima K, Iseki E, Murayama S, Kretzschmar H, Neumann M, Lippa C, Halliday G, MacKenzie J, Ravid R, Dickson D, Wszolek Z, Iwatsubo T, Pickering-Brown SM, Holton J, Lees A, Revesz T, Mann DM. An immunohistochemical study of cases of sporadic and inherited frontotemporal lobar degeneration using 3R- and 4R-specific tau monoclonal antibodies. Acta Neuropathol. 2006;111(4):329–340. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

