Innate and adaptive immune responses against Staphylococcus aureus skin infections
- PMID: 22057887
- PMCID: PMC5937532
- DOI: 10.1007/s00281-011-0292-6
Innate and adaptive immune responses against Staphylococcus aureus skin infections
Abstract
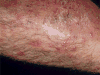
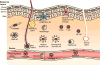
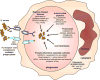
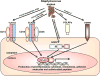
Staphylococcus aureus is an important human pathogen that is responsible for the vast majority of bacterial skin and soft tissue infections in humans. S. aureus can also become more invasive and cause life-threatening infections such as bacteremia, pneumonia, abscesses of various organs, meningitis, osteomyelitis, endocarditis, and sepsis. These infections represent a major public health threat due to the enormous numbers of these infections and the widespread emergence of methicillin-resistant S. aureus (MRSA) strains. MSRA is endemic in hospitals worldwide and is rapidly spreading throughout the normal human population in the community. The increasing frequency of MRSA infections has complicated treatment as these strains are more virulent and are increasingly becoming resistant to multiple different classes of antibiotics. The important role of the immune response against S. aureus infections cannot be overemphasized as humans with certain genetic and acquired immunodeficiency disorders are at an increased risk for infection. Understanding the cutaneous immune responses against S. aureus is essential as most of these infections occur or originate from a site of infection or colonization of the skin and mucosa. This review will summarize the innate immune responses against S. aureus skin infections, including antimicrobial peptides that have direct antimicrobial activity against S. aureus as well as pattern recognition receptors and proinflammatory cytokines that promote neutrophil abscess formation in the skin, which is required for bacterial clearance. Finally, we will discuss the recent discoveries involving IL-17-mediated responses, which provide a key link between cutaneous innate and adaptive immune responses against S. aureus skin infections.
Figures
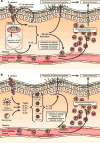
References
-
- Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, Carey RB, Talan DA. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666–674. - PubMed
-
- Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–1771. - PubMed
-
- Daum RS. Clinical practice. Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N Engl J Med. 2007;357:380–390. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

