Simvastatin promotes osteogenic differentiation of mouse embryonic stem cells via canonical Wnt/β-catenin signaling
- PMID: 22058016
- PMCID: PMC3887698
- DOI: 10.1007/s10059-011-0107-6
Simvastatin promotes osteogenic differentiation of mouse embryonic stem cells via canonical Wnt/β-catenin signaling
Abstract
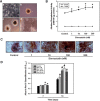
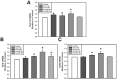
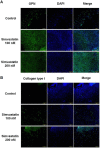
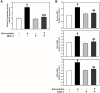
Simvastatin, an inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, has been known to reduce cholesterol biosynthesis. However, recent studies demonstrate that simvastatin shows diverse cholesterol-independent functions including cellular differentiation. In this study, we investigated the stimulatory effect of simvastatin on the osteogenic differentiation of mouse embryonic stem cells (ESCs). The osteogenic effect of simvastatin was observed at relatively low doses (ranging from 1 nM to 200 nM). Incubation of ESCs in simvastatin-supplemented osteogenic medium significantly increased alkaline phosphatase (ALP) activity at day 7. The matrix mineralization was also augmented and demonstrated pivotal levels after 14 days incubation of simvastatin. Osteogenic differentiation of ESCs by simvastatin was determined by upregulation of the mRNA expression of runtrelated gene 2 (Runx2), osterix (OSX), and osteocalcin (OCN) as osteogenic transcription factors. Moreover, the increased protein expression of OCN, osteopontin (OPN), and collagen type I (Coll I) was assessed using Western blot analysis and immunocytochemistry. However, the blockage of canonical Wnt signaling by DKK-1 downregulated simvastatin-induced ALP activity and the mRNA expression of each osteogenic transcription factor. Furthermore, the β-catenin specific siRNA transfection decreased the protein levels of OCN, OPN, and Coll I. Collectively, these findings suggest that simvastatin enhances the differentiation of ESCs toward osteogenic lineage through activation of canonical Wnt/β-catenin signaling.
Figures


References
-
- Bielby R.C., Boccaccini A.R., Polak J.M., Buttery L.D. In vitro differentiation and in vivo mineralization of osteogenic cells derived from human embryonic stem cells. Tissue Eng. (2004);10:1518–1525. - PubMed
-
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. (1976);72:248–254. - PubMed
-
- Buttery L.D., Bourne S., Xynos J.D., Wood H., Hughes F.J., Hughes S.P., Episkopou V., Polak J.M. Differentiation of osteoblasts and in vitro bone formation from murine embryonic stem cells. Tissue Eng. (2001);7:89–99. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

