Cyclophosphamide, thalidomide, and dexamethasone as induction therapy for newly diagnosed multiple myeloma patients destined for autologous stem-cell transplantation: MRC Myeloma IX randomized trial results
- PMID: 22058209
- PMCID: PMC3291601
- DOI: 10.3324/haematol.2011.043372
Cyclophosphamide, thalidomide, and dexamethasone as induction therapy for newly diagnosed multiple myeloma patients destined for autologous stem-cell transplantation: MRC Myeloma IX randomized trial results
Abstract
Background: Thalidomide is active in multiple myeloma and is associated with minimal myelosuppression, making it a good candidate for induction therapy prior to high-dose therapy with autologous stem-cell transplantation.
Design and methods: Oral cyclophosphamide, thalidomide, and dexamethasone was compared with infusional cyclophosphamide, vincristine, doxorubicin, and dexamethasone in patients with newly diagnosed multiple myeloma.
Results: The post-induction overall response rate (≥ partial response) for the intent-to-treat population was significantly higher with cyclophosphamide-thalidomide-dexamethasone (n=555) versus cyclophosphamide-vincristine-doxorubicin-dexamethasone (n=556); 82.5% versus 71.2%; odds ratio 1.91; 95% confidence interval 1.44-2.55; P<0.0001. The complete response rates were 13.0% with cyclophosphamide-thalidomide-dexamethasone and 8.1% with cyclophos-phamide-vincristine-doxorubicin-dexamethasone (P=0.0083), with this differential response being maintained in patients who received autologous stem-cell transplantation (post-transplant complete response 50.0% versus 37.2%, respectively; P=0.00052). Cyclophosphamide-thalidomide-dexamethasone was non-inferior to cyclophosphamide-vincristine-doxorubicin-dexamethasone for progression-free and overall survival, and there was a trend toward a late survival benefit with cyclophosphamide-thalidomide-dexamethasone in responders. A trend toward an overall survival advantage for cyclophosphamide-thalidomide-dexamethasone over cyclophosphamide-vincristine-doxorubicin-dexamethasone was also observed in a subgroup of patients with favorable interphase fluorescence in situ hybridization. Compared with cyclophosphamide-vincristine-doxorubicin-dexamethasone, cyclophosphamide-thalidomide-dexamethasone was associated with more constipation and somnolence, but a lower incidence of cytopenias.
Conclusions: The cyclophosphamide-thalidomide-dexamethasone regimen showed improved response rates and was not inferior in terms of survival outcomes to the standard infusional regimen of cyclophosphamide-vincristine-doxorubicin-dexamethasone. Based on its oral administration and the reduced incidence of infection and cytopenia, cyclophosphamide-thalidomide-dexa-methasone may be considered an effective induction therapy option for patients with newly diagnosed multiple myeloma. (ISRCTN: 68454111).
Figures

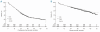
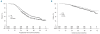

References
-
- Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Français du Myélome. N Engl J Med. 1996;335(2):91–7. - PubMed
-
- Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348(19):1875–83. - PubMed
-
- Rajkumar SV, Blood E, Vesole D, Fonseca R, Greipp PR Eastern Cooperative Oncology Group. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2006;24(3):431–6. - PubMed
-
- Rajkumar SV, Rosiñol L, Hussein M, Catalano J, Jedrzejczak W, Lucy L, et al. Multicenter, randomized, double-blind, placebo-controlled study of thalidomide plus dexamethasone compared with dexamethasone as initial therapy for newly diagnosed multiple myeloma. J Clin Oncol. 2008;26(13):2171–7. - PMC - PubMed
-
- Hulin C, Facon T, Rodon P, Pegourie B, Benboubker L, Doyen C, et al. Efficacy of melphalan and prednisone plus thalidomide in patients older than 75 years with newly diagnosed multiple myeloma: IFM 01/01 trial. J Clin Oncol. 2009;27(22):3664–70. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

