Mussel-Inspired Adhesives and Coatings
- PMID: 22058660
- PMCID: PMC3207216
- DOI: 10.1146/annurev-matsci-062910-100429
Mussel-Inspired Adhesives and Coatings
Abstract
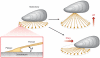
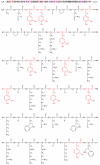
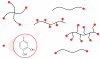
Mussels attach to solid surfaces in the sea. Their adhesion must be rapid, strong, and tough, or else they will be dislodged and dashed to pieces by the next incoming wave. Given the dearth of synthetic adhesives for wet polar surfaces, much effort has been directed to characterizing and mimicking essential features of the adhesive chemistry practiced by mussels. Studies of these organisms have uncovered important adaptive strategies that help to circumvent the high dielectric and solvation properties of water that typically frustrate adhesion. In a chemical vein, the adhesive proteins of mussels are heavily decorated with Dopa, a catecholic functionality. Various synthetic polymers have been functionalized with catechols to provide diverse adhesive, sealant, coating, and anchoring properties, particularly for critical biomedical applications.
Figures







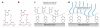





References
-
- Comyn J. The relationship between joint durability and water diffusion. In: Kinloch AJ, editor. Developments in Adhesives. Vol. 2. Appl. Sci. Publ.; Barking, UK: 1981. pp. 279–313.
-
- Israelachvili JN. Intermolecular and Surface Forces. 3rd ed Elsevier; London: 2010.
-
- Gutmann V. Solvent effects on the reactivities of organometallic compounds. Coord. Chem. Rev. 1976;18:225–55.
-
- Clint JH, Wicks AC. Adhesion under water: surface energy considerations. Int. J. Adhes. Adhes. 2001;21:267–73.
-
- Pocius AV. Adhesives and Adhesives Technology. Hanser/Gardner Publ.; Cincinnati: 1997.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
