Engineering Parvalbumin for the Heart: Optimizing the Mg Binding Properties of Rat β-Parvalbumin
- PMID: 22059076
- PMCID: PMC3204457
- DOI: 10.3389/fphys.2011.00077
Engineering Parvalbumin for the Heart: Optimizing the Mg Binding Properties of Rat β-Parvalbumin
Abstract
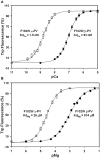
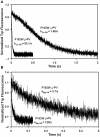
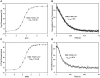
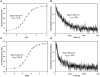
Parvalbumin (PV), an EF-hand protein family member, is a delayed calcium buffer that exchanges magnesium for calcium to facilitate fast skeletal muscle relaxation. Genetic approaches that express parvalbumin in the heart also enhance relaxation and show promise of being therapeutic against various cardiac diseases where relaxation is compromised. Unfortunately, skeletal muscle PVs have very slow rates of Ca(2+) dissociation and are prone to becoming saturated with Ca(2+), eventually losing their buffering capability within the constantly beating heart. In order for PV to have a more therapeutic potential in the heart, a PV with faster rates of calcium dissociation and high Mg(2+) affinity is needed. We demonstrate that at 35°C, rat β-PV has an ~30-fold faster rate of Ca(2+) dissociation compared to rat skeletal muscle α-PV, and still possesses a physiologically relevant Ca(2+) affinity (~100 nM). However, rat β-PV will not be a delayed Ca(2+) buffer since its Mg(2+) affinity is too low (~1 mM). We have engineered two mutations into rat β-PV, S55D and E62D, when observed alone increase Mg(2+) affinity up to fivefold, but when combined increase Mg(2+) affinity ~13-fold, well within a physiologically relevant affinity. Furthermore, the Mg(2+) dissociation rate (172/s) from the engineered S55D, E62D PV is slow enough for delayed Ca(2+) buffering. Additionally, the engineered PV retains a high Ca(2+) affinity (132 nM) and fast rate of Ca(2+) dissociation (64/s). These PV design strategies hold promise for the development of new therapies to remediate relaxation abnormalities in different heart diseases and heart failure.
Keywords: calcium; magnesium; parvalbumin; relaxation.
Figures

References
-
- Csillik B., Schwaller B., Mihaly A., Henzi T., Losonczi E., Knyihar-Csillik E. (2010). Upregulated expression of oncomodulin, the beta isoform of parvalbumin, in perikarya and axons in the diencephalon of parvalbumin knockout mice. Neuroscience 165, 749–75710.1016/j.neuroscience.2009.10.048 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

