A randomised controlled trial of probiotics for the prevention of spontaneous preterm delivery associated with bacterial vaginosis: preliminary results
- PMID: 22059409
- PMCID: PMC3264514
- DOI: 10.1186/1745-6215-12-239
A randomised controlled trial of probiotics for the prevention of spontaneous preterm delivery associated with bacterial vaginosis: preliminary results
Abstract
Background: Bacterial vaginosis increases the risk of spontaneous preterm delivery at less than 34 weeks of gestation.
Objective: The purpose of this study was to evaluate the efficacy of the early administration of selected lactobacilli strains (probiotics) to pregnant women with asymptomatic bacterial vaginosis/intermediate-degree infections to prevent spontaneous premature delivery and associated neonatal morbidity.
Methods/design: Asymptomatic pregnant women at less than 20 weeks of gestation, with no indication of elective preterm delivery, with a vaginal pH ≥ 4.5 and Nugent score > 3 were randomly assigned to the placebo or intervention group (oral administration of selected lactobacilli up to the 24th to 26th week of gestation). The randomisation was stratified for the history of premature delivery (HPD) and blocked. The allocation was concealed, and the participating health professionals and patients were blinded. The primary outcome was preterm delivery (<34 to <32 weeks), and the secondary outcomes were associated neonatal complications.
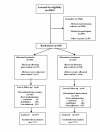
Results: In total, 4,204 pregnant women were screened; 320 and 324 individuals were respectively randomly assigned to the placebo and intervention groups, and 62% finished the trial. None of the randomised patients were lost to follow-up. For the non-HPD stratum, the intent-to-treat relative risks of spontaneous premature birth at < 34 and < 37 weeks' gestation were 0.33 (0.03, 3.16) and 0.49 (0.17, 1.44), respectively, and they were non-significant (ns) with p = 0.31 and 0.14. The corresponding actual treatment figures were zero and 0.32 (0.09, 1.19), which were ns with p = 0.12 and 0.06. The intent-to-treat relative risk of spontaneous premature birth at < 37 weeks of gestation for the trial as a whole, including HPD and non-HPD participants, was 0.69 (0.26, 1.78), p = 0.30 (ns). The neonatal complications under evaluation occurred in only one infant (< 34 weeks; placebo group) who presented with respiratory distress syndrome and suspected early neonatal sepsis. The recorded adverse events were minor and relatively non-specific.
Conclusions: The efficacy of the tested probiotics to prevent preterm delivery among women without a history of preterm delivery was not determined because the study sample was insufficient to estimate statistically significant intent-to-treat effects; additional studies are needed to evaluate this intervention among these women.
Trial registration: Trial registration at NIH register: NCT00303082. Sources of funding: the Brazilian Health Ministry and the State of Rio de Janeiro Research Foundation.
Figures
References
-
- Agency for Healthcare Research and Quality. Management of preterm labor. Evidence report/technology assessment n. 18, AHRQ public n. 01-E021. 2000.
-
- Leal MC, Gama SGN, Campos MR. Fatores associados à morbi-mortalidade perinatal em uma amostra de maternidades públicas e privadas do município do Rio de Janeiro, 1999-2001. Cadernos de Saúde Pública. 2004;20(Supl 1):S20–S33. - PubMed
-
- Campos MR, Leal MC, Souza PR Jr, Cunha CB. Consistência entre fontes de dados e confiabilidade inter-observador do estudo da morbi-mortalidade e atenção peri e neonatal no município do Rio de Janeiro. Cadernos de Saúde Pública. 2004;20(Supl 1):S34–S43. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

