Mass-dependent bond vibrational dynamics influence catalysis by HIV-1 protease
- PMID: 22059645
- PMCID: PMC3227791
- DOI: 10.1021/ja209391n
Mass-dependent bond vibrational dynamics influence catalysis by HIV-1 protease
Abstract
Protein motions that occur on the microsecond to millisecond time scale have been linked to enzymatic rates observed for catalytic turnovers, but not to transition-state barrier crossing. It has been hypothesized that enzyme motions on the femtosecond time scale of bond vibrations play a role in transition state formation. Here, we perturb femtosecond motion by substituting all nonexchangeable carbon, nitrogen, and hydrogen atoms with (13)C, (15)N, and (2)H and observe the catalytic effects in HIV-1 protease. According to the Born-Oppenheimer approximation, isotopic substitution alters vibrational frequency with unchanged electrostatic properties. With the use of a fluorescent peptide to report on multiple steps in the reaction, we observe significantly reduced rates in the heavy enzyme relative to the light enzyme. A possible interpretation of our results is that there exists a dynamic link between mass-dependent bond vibrations of the enzyme and events in the reaction coordinate.
© 2011 American Chemical Society
Figures



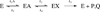
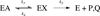
References
-
- Wolfenden R, Snider MJ. Accounts Chem Res. 2001;34:938. - PubMed
-
- Hammes-Schiffer S, Benkovic SJ. Annu Rev Biochem. 2006;75:519. - PubMed
-
- Boehr DD, Dyson HJ, Wright PE. Chem Rev. 2006;106:3055. - PubMed
-
- Henzler-Wildman KA, Thai V, Lei M, Ott M, Wolf-Watz M, Fenn T, Pozharski E, Wilson MA, Petsko GA, Karplus M, Hubner CG, Kern D. Nature. 2007;450:838. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

