Inhibition of prolyl hydroxylases by dimethyloxaloylglycine after stroke reduces ischemic brain injury and requires hypoxia inducible factor-1α
- PMID: 22061780
- PMCID: PMC3286647
- DOI: 10.1016/j.nbd.2011.10.020
Inhibition of prolyl hydroxylases by dimethyloxaloylglycine after stroke reduces ischemic brain injury and requires hypoxia inducible factor-1α
Abstract
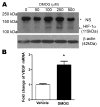
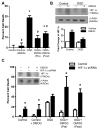
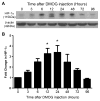
Pathological oxygen deprivation inhibits prolyl hydroxylase (PHD) activity and stimulates a protective cellular oxygen-sensing response in part through the stabilization and activation of the Hypoxia Inducible Factor (HIF) 1α transcription factor. The present investigation tested the therapeutic potential of enhanced activation of oxygen-sensing pathways by competitive pharmacologic PHD inhibition after stroke, hypothesizing that post-ischemic PHD inhibition would reduce neuronal cell death and require the activation of HIF-1α. The PHD inhibitor dimethyloxaloylglycine (DMOG, 100 μM) reduced cell death by oxygen glucose deprivation (OGD), an in vitro model of ischemia, and the protection required HIF-1α. In vivo, DMOG (50 mg/kg, i.p.) administered 30 or 60 min after distal occlusion of the middle cerebral artery (MCA) in mice enhanced the activation of HIF-1α protein, enhanced transcription of the HIF-regulated genes vascular endothelial growth factor, erythropoietin, endothelial nitric oxide synthase, and pyruvate dehydrogenase kinase-1, reduced ischemic infarct volume and activation of the pro-apoptotic caspase-3 protein, reduced behavioral deficits after stroke, and reduced the loss of local blood flow in the MCA territory after stroke. Inhibition of HIF-1α in vivo by Digoxin or Acriflavine abrogated the infarct sparing properties of DMOG. These data suggest that supplemental activation of oxygen-sensing pathways after stroke may provide a clinically applicable intervention for the promotion of neurovascular cell survival after ischemia.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures



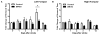

References
-
- Abu-Amara M, Yang SY, Quaglia A, Rowley P, Fuller B, Seifalian A, Davidson B. Role of endothelial nitric oxide synthase in remote ischemic preconditioning of the mouse liver. Liver Transpl. 2011;17:610–619. - PubMed
-
- American Heart Association. Heart Disease and Stroke Statistics. Dallas, Texas, USA: American Heart Association; (2008 Update)
-
- Aminova LR, Chavez JC, Lee J, Ryu H, Kung A, Lamanna JC, Ratan RR. Prosurvival and prodeath effects of hypoxia-inducible factor-1alpha stabilization in a murine hippocampal cell line. J Biol Chem. 2005;280:3996–4003. - PubMed
-
- Aragones J, et al. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat Genet. 2008;40:170–180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

