Distinct cellular pools of perilipin 5 point to roles in lipid trafficking
- PMID: 22063271
- PMCID: PMC3740563
- DOI: 10.1016/j.bbalip.2011.10.017
Distinct cellular pools of perilipin 5 point to roles in lipid trafficking
Abstract
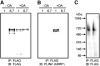
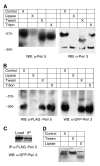
The PAT family of lipid storage droplet proteins comprised five members, each of which has become an established regulator of cellular neutral lipid metabolism. Perilipin 5 (also known as lsdp-5, MLDP, PAT-1, and OXPAT), the most recently discovered member of the family, has been shown to localize to two distinct intracellular pools: the lipid storage droplet (LD), and a poorly characterized cytosolic fraction. We have characterized the denser of these intracellular pools and find that a population of perilipin 5 not associated with large LDs resides in complexes with a discrete density (~1.15 g/ml) and size (~575 kDa). Using immunofluorescence, western blotting of isolated sucrose density fractions, native gradient gel electrophoresis, and co-immunoprecipitation, we have shown that these small (~15 nm), perilipin 5-encoated structures do not contain the PAT protein perilipin 2 (ADRP), but do contain perilipin 3 and several other as of yet uncharacterized proteins. The size and density of these particles as well as their susceptibility to degradation by lipases suggest that like larger LDs, they have a neutral lipid rich core. When treated with oleic acid to promote neutral lipid deposition, cells ectopically expressing perilipin 5 experienced a reorganization of LDs in the cell, resulting in fewer, larger droplets at the expense of smaller ones. Collectively, these data demonstrate that a portion of cytosolic perilipin 5 resides in high density lipid droplet complexes that participate in cellular neutral lipid accumulation.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures






References
-
- Cowie CC, Engelgau MM, Rust KF, Saydah SH, Byrd-Holt DD, Williams DE, Eberhardt MS, Geiss LS, Flegal KM, Gregg EW. Prevalence of diabetes and impaired fasting glucose in adults in the US population—National Health and Nutrition Examination Survey 1999–2002. Diabetes Care. 2006;29:1263–1268. - PubMed
-
- Bostrom P, Andersson L, Rutberg M, Perman J, Lidberg U, Johansson BR, Fernandez-Rodriguez J, Ericson J, Nilsson T, Boren J, Olofsson SO. SNARE proteins mediate fusion between cytosolic lipid droplets and are implicated in insulin sensitivity. Nat. Cell Biol. 2007;9:1286–1293. - PubMed
-
- Bostrom P, Andersson L, Li L, Perkins R, Hojlund K, Boren J, Olofsson SO. The assembly of lipid droplets and its relation to cellular insulin sensitivity. Biochem. Soc. Trans. 2009;37:981–985. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

