Human sympathetic outflows to skin and muscle target organs fluctuate concordantly over a wide range of time-varying frequencies
- PMID: 22063627
- PMCID: PMC3285071
- DOI: 10.1113/jphysiol.2011.214528
Human sympathetic outflows to skin and muscle target organs fluctuate concordantly over a wide range of time-varying frequencies
Abstract
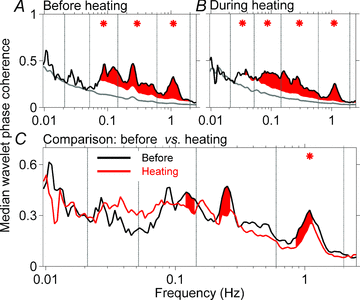
Frequency-domain analyses of simultaneously recorded skin and muscle sympathetic nerve activities may yield unique information on otherwise obscure central processes governing human neural outflows. We used wavelet transform and wavelet phase coherence methods to analyse integrated skin and muscle sympathetic nerve activities and haemodynamic fluctuations, recorded from nine healthy supine young men. We tested two null hypotheses: (1) that human skin and muscle sympathetic nerve activities oscillate congruently; and (2) that whole-body heating affects these neural outflows and their haemodynamic consequences in similar ways. Measurements included peroneal nerve skin and tibial nerve muscle sympathetic activities; the electrocardiogram; finger photoplethysmographic arterial pressure; respiration (controlled at 0.25 Hz, and registered with a nasal thermistor); and skin temperature, sweating, and laser-Doppler skin blood flow. We made recordings at ∼27°C, for ∼20 min, and then during room temperature increases to ∼38°C, over 35 min. We analysed data with a wavelet transform, using the Morlet mother wavelet and wavelet phase coherence, to determine the frequencies and coherences of oscillations over time. At 27°C, skin and muscle nerve activities oscillated coherently, at ever-changing frequencies between 0.01 and the cardiac frequency (∼1 Hz). Heating significantly augmented oscillations of skin sympathetic nerve activity and skin blood flow, arterial pressure, and R-R intervals, over a wide range of low frequencies, and modestly reduced coordination between skin and muscle sympathetic oscillations. These results suggest that human skin and muscle sympathetic motoneurones are similarly entrained by external influences, including those of arterial baroreceptors, respiration, and other less well-defined brainstem oscillators. Our study provides strong support for the existence of multiple, time-varying central sympathetic neural oscillators in human subjects.
Figures







References
-
- Akselrod S, Gordon D, Ubel FA, Shannon DC, Barger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213:220–222. - PubMed
-
- Badra LJ, Cooke WH, Hoag JB, Crossman AA, Kuusela TA, Tahvanainen KUO, Eckberg DL. Respiratory modulation of human autonomic rhythms. Am J Physiol Heart Circ Physiol. 2001;280:H2674–H2688. - PubMed
-
- Bandrivskyy A, Bernjak A, McClintock P, Stefanovska A. Wavelet phase coherence analysis: application to skin temperature and blood flow. Cardiovasc Eng. 2004;4:89–93.
-
- Barman SM, Gebber GL. Subgroups of rostral ventrolateral medullary and caudal medullary raphe neurons based on patterns of relationship to sympathetic nerve discharge and axonal projections. J Neurophysiol. 1997;77:65–75. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

