Developmentally regulated SCN5A splice variant potentiates dysfunction of a novel mutation associated with severe fetal arrhythmia
- PMID: 22064211
- PMCID: PMC3292693
- DOI: 10.1016/j.hrthm.2011.11.006
Developmentally regulated SCN5A splice variant potentiates dysfunction of a novel mutation associated with severe fetal arrhythmia
Abstract
Background: Congenital long-QT syndrome (LQTS) may present during fetal development and can be life-threatening. The molecular mechanism for the unusual early onset of LQTS during fetal development is unknown.
Objective: We sought to elucidate the molecular basis for severe fetal LQTS presenting at 19 weeks' gestation, the earliest known presentation of this disease.
Methods: Fetal magnetocardiography was used to demonstrated torsades de pointes and a prolonged rate-corrected QT interval. In vitro electrophysiological studies were performed to determine functional consequences of a novel SCN5A mutation found in the fetus.
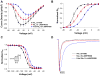
Results: The fetus presented with episodes of ventricular ectopy progressing to incessant ventricular tachycardia and hydrops fetalis. Genetic analysis disclosed a novel, de novo heterozygous mutation (L409P) and a homozygous common variant (R558 in SCN5A). In vitro electrophysiological studies demonstrated that the mutation in combination with R558 caused significant depolarized shifts in the voltage dependence of inactivation and activation, faster recovery from inactivation, and a 7-fold higher level of persistent current. When the mutation was engineered in a fetal-expressed SCN5A splice isoform, channel dysfunction was markedly potentiated. Also, R558 alone in the fetal splice isoform evoked a large persistent current, and hence both alleles were dysfunctional.
Conclusion: We report the earliest confirmed diagnosis of symptomatic LQTS and present evidence that mutant cardiac sodium channel dysfunction is potentiated by a developmentally regulated alternative splicing event in SCN5A. Our findings provide a plausible mechanism for the unusual severity and early onset of cardiac arrhythmia in fetal LQTS.
Copyright © 2012 Heart Rhythm Society. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of Interest: None
Figures





Comment in
-
Enhanced impact of SCN5A mutation associated with long QT syndrome in fetal splice isoform.Heart Rhythm. 2012 Apr;9(4):598-9. doi: 10.1016/j.hrthm.2011.11.049. Epub 2011 Nov 30. Heart Rhythm. 2012. PMID: 22138134 Free PMC article. No abstract available.
References
-
- Schwartz PJ, Crotti L. Long QT and short QT syndrome. In: Zipes DP, Jalife J, editors. Cardiac Electrophysiology: From Cell to Bedside. Fifth. Philadelphia: Elsevier/Saunders; 2009. pp. 731–744.
-
- Wedekind H, Smits JP, Schulze-Bahr E, et al. De novo mutation in the SCN5A gene associated with early onset of sudden infant death. Circulation. 2001;104:1158–1164. - PubMed
-
- Schwartz PJ, Priori SG, Dumaine R, et al. A molecular link between the sudden infant death syndrome and the long-QT syndrome. N Engl J Med. 2000;343:262–267. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

