The neutrophil in vascular inflammation
- PMID: 22064428
- PMCID: PMC7095830
- DOI: 10.1038/nm.2514
The neutrophil in vascular inflammation
Abstract
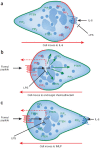
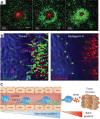
Here we focus on how neutrophils have a key regulatory role in vascular inflammation. Recent studies using advanced imaging techniques have yielded new insights into the mechanisms by which neutrophils contribute to defense against bacterial infections and also against sterile injury. In these settings, neutrophils are recruited by various mechanisms depending on the situation. We also describe how these processes may be disrupted in systemic infections, with a particular emphasis on mouse models of sepsis. Neutrophils are often immobilized in the lungs and liver during systemic infections, and this immobilization may be a mechanism through which bacteria can evade the innate immune response or allow neutrophils to form neutrophil extracellular traps that trap and kill bacteria in blood. The platelet is also an important player in sepsis, and we describe how it collaborates with neutrophils in the formation of neutrophil extracellular traps.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Auffray C, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. - PubMed
-
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007;7:678–689. - PubMed
-
- Petri B, Phillipson M, Kubes P. The physiology of leukocyte recruitment: an in vivo perspective. J. Immunol. 2008;180:6439–6446. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

