A U1-U2 snRNP interaction network during intron definition
- PMID: 22064476
- PMCID: PMC3255776
- DOI: 10.1128/MCB.06234-11
A U1-U2 snRNP interaction network during intron definition
Abstract
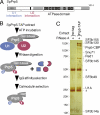
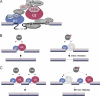
The assembly of prespliceosomes is responsible for selection of intron sites for splicing. U1 and U2 snRNPs recognize 5' splice sites and branch sites, respectively; although there is information regarding the composition of these complexes, little is known about interaction among the components or between the two snRNPs. Here we describe the protein network of interactions linking U1 and U2 snRNPs with the ATPase Prp5, important for branch site recognition and fidelity during the first steps of the reaction, using fission yeast Schizosaccharomyces pombe. The U1 snRNP core protein U1A binds to a novel SR-like protein, Rsd1, which has homologs implicated in transcription. Rsd1 also contacts S. pombe Prp5 (SpPrp5), mediated by SR-like domains in both proteins. SpPrp5 then contacts U2 snRNP through SF3b, mediated by a conserved DPLD motif in Prp5. We show that mutations in this motif have consequences not only in vitro (defects in prespliceosome formation) but also in vivo, yielding intron retention and exon skipping defects in fission yeast and altered intron recognition in budding yeast Saccharomyces cerevisiae, indicating that the U1-U2 network provides critical, evolutionarily conserved contacts during intron definition.
Figures


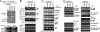

References
-
- Abovich N, Rosbash M. 1997. Cross-intron bridging interactions in the yeast commitment complex are conserved in mammals. Cell 89:403–412 - PubMed
-
- Bahler J, et al. 1998. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14:943–951. - PubMed
-
- Barabino SM, Blencowe BJ, Ryder U, Sproat BS, Lamond AI. 1990. Targeted snRNP depletion reveals an additional role for mammalian U1 snRNP in spliceosome assembly. Cell 63:293–302 - PubMed
-
- Berget SM. 1995. Exon recognition in vertebrate splicing. J. Biol. Chem. 270:2411–2414 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
