A history of hookworm vaccine development
- PMID: 22064562
- PMCID: PMC3323499
- DOI: 10.4161/hv.7.11.18443
A history of hookworm vaccine development
Abstract
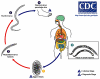
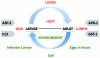
The human hookworms Necator americanus and Ancylostoma duodenale remain among the most common infections of humans in areas of rural poverty in the developing regions of the world, with an estimated 1 billion people infected with one or more of these parasites. Herein, we review the nearly 100 years of research, development, animal testing, and fieldwork that have led to our current progress in recombinant hookworm vaccines. We begin with the identification of hookworm at the start of the 20th century in Southern US, then discuss the progress in developed countries to eliminate human hookworm infection, and then the industrial development and field use in the 1970s a canine hookworm vaccine(Ancylostoma caninum), and finally our progress to date in the development and clinical testing of an array of recombinant antigens to prevent human hookworm disease from N. americanus infection. Special attention is given to the challenges faced in the development of a vaccine against a blood-feeding nematode, including the epidemiology of infection (high prevalence of infection), pathogenesis (chronic infection that increases with the age of the host), and a robust immune response that fails to confer the protection in the host and a concomitant absence of correlates of protection by a successful vaccine could be developed and tested. Finally, we provide the optimal and acceptable profiles of a human hookworm vaccine, including the proposed indication, target population, and route of administration, as developed by the Human Hookworm Vaccine Initiative, the only group currently working on vaccines targeting this parasite.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
