The 5α-androstanedione pathway to dihydrotestosterone in castration-resistant prostate cancer
- PMID: 22064602
- PMCID: PMC3262939
- DOI: 10.2310/JIM.0b013e31823874a4
The 5α-androstanedione pathway to dihydrotestosterone in castration-resistant prostate cancer
Abstract
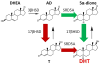
The survival and progression of prostate cancer are generally dependent on expression of the androgen receptor (AR), as well as the availability of endogenous AR agonists. Originating from the gonads, testosterone is released into circulation and is converted by steroid-5α-reductase in prostate cancer to 5α-dihydrotestosterone (DHT), potently activating AR and driving tumor progression. Advanced prostate cancer is initially treated with gonadal testosterone depletion, which suppresses this cascade of events and typically leads to a treatment response. Eventually, resistance to testosterone deprivation occurs with "castration-resistant" prostate cancer (CRPC) and is driven by the intratumoral synthesis of DHT. The generation of DHT occurs in large part from adrenal 19-carbon precursor steroids, which are dependent on expression of CYP17A1. Although the path from adrenal precursor steroids to DHT was generally thought to require 5α-reduction of testosterone, recent data suggest that it instead involves conversion from Δ-androstenedione by steroid-5α-reductase isoenzyme-1 to 5α-androstanedione, followed by subsequent conversion to DHT. The 5α-androstanedione pathway to DHT therefore bypasses testosterone entirely. Abiraterone acetate effectively inhibits CYP17A1, blocks the synthesis of androgens, and extends the survival of men with CRPC. Further progress in the hormonal treatment of CRPC is dependent on an understanding of the mechanisms that underlie CRPC and resistance to abiraterone acetate.
Figures
References
-
- Tomlins SA, et al. Integrative molecular concept modeling of prostate cancer progression. Nat Genet. 2007;39:41–51. - PubMed
-
- Attard G, et al. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Res. 2009;69:2912–2918. - PubMed
-
- Deslypere JP, Young M, Wilson JD, McPhaul MJ. Testosterone and 5 alpha-dihydrotestosterone interact differently with the androgen receptor to enhance transcription of the MMTV-CAT reporter gene. Mol Cell Endocrinol. 1992;88:15–22. - PubMed
-
- Bruchovsky N, Wilson JD. The intranuclear binding of testosterone and 5-alpha-androstan-17-beta-ol-3-one by rat prostate. J Biol Chem. 1968;243:5953–5960. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

