Combined roles of human IgG subclass, alternative complement pathway activation, and epitope density in the bactericidal activity of antibodies to meningococcal factor h binding protein
- PMID: 22064712
- PMCID: PMC3255668
- DOI: 10.1128/IAI.05956-11
Combined roles of human IgG subclass, alternative complement pathway activation, and epitope density in the bactericidal activity of antibodies to meningococcal factor h binding protein
Abstract
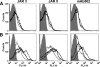
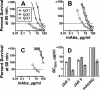
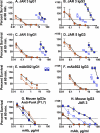
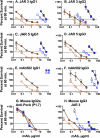
Meningococcal vaccines containing factor H binding protein (fHbp) are in clinical development. fHbp binds human fH, which enables the meningococcus to resist complement-mediated bacteriolysis. Previously, we found that chimeric human IgG1 mouse anti-fHbp monoclonal antibodies (MAbs) had human complement-mediated bactericidal activity only if the MAb inhibited fH binding. Since IgG subclasses differ in their ability to activate complement, we investigated the role of human IgG subclasses on antibody functional activity. We constructed chimeric MAbs in which three different murine fHbp-specific binding domains were each paired with human IgG1, IgG2, or IgG3. Against a wild-type group B isolate, all three IgG3 MAbs, irrespective of their ability to inhibit fH binding, had bactericidal activity that was >5-fold higher than the respective IgG1 MAbs, while the IgG2 MAbs had the least activity. Against a mutant with increased fHbp expression, the anti-fHbp MAbs elicited greater C4b deposition (classical pathway) and greater bactericidal activity than against the wild-type strain, and the IgG1 MAbs had similar or greater activity than the respective IgG3 MAbs. The bactericidal activity against both wild-type and mutant strains also was dependent, in part, on activation of the alternative complement pathway. Thus, at lower epitope density in the wild-type strain, the IgG3 anti-fHbp MAbs had the greatest bactericidal activity. At a higher epitope density in the mutant, the IgG1 MAbs had similar or greater bactericidal activity than the IgG3 MAbs, and the activity was less dependent on the inhibition of fH binding than at a lower epitope density.
Figures
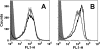


Similar articles
-
Complement-mediated bactericidal activity of anti-factor H binding protein monoclonal antibodies against the meningococcus relies upon blocking factor H binding.Infect Immun. 2011 Sep;79(9):3751-9. doi: 10.1128/IAI.05182-11. Epub 2011 Jun 27. Infect Immun. 2011. PMID: 21708990 Free PMC article.
-
Monoclonal antibodies to meningococcal factor H binding protein with overlapping epitopes and discordant functional activity.PLoS One. 2012;7(3):e34272. doi: 10.1371/journal.pone.0034272. Epub 2012 Mar 26. PLoS One. 2012. PMID: 22461909 Free PMC article.
-
Complement-dependent synergistic bactericidal activity of antibodies against factor H-binding protein, a sparsely distributed meningococcal vaccine antigen.J Infect Dis. 2008 Apr 1;197(7):1053-61. doi: 10.1086/528994. J Infect Dis. 2008. PMID: 18419542
-
Does binding of complement factor H to the meningococcal vaccine antigen, factor H binding protein, decrease protective serum antibody responses?Clin Vaccine Immunol. 2013 Aug;20(8):1099-107. doi: 10.1128/CVI.00260-13. Epub 2013 Jun 5. Clin Vaccine Immunol. 2013. PMID: 23740919 Free PMC article. Review.
-
Meningococcal factor H binding protein as immune evasion factor and vaccine antigen.FEBS Lett. 2020 Aug;594(16):2657-2669. doi: 10.1002/1873-3468.13793. Epub 2020 May 12. FEBS Lett. 2020. PMID: 32298465 Review.
Cited by
-
Investigation into the Antigenic Properties and Contributions to Growth in Blood of the Meningococcal Haemoglobin Receptors, HpuAB and HmbR.PLoS One. 2015 Jul 24;10(7):e0133855. doi: 10.1371/journal.pone.0133855. eCollection 2015. PLoS One. 2015. PMID: 26208277 Free PMC article.
-
4CMenB vaccine induces elite cross-protective human antibodies that compete with human factor H for binding to meningococcal fHbp.PLoS Pathog. 2020 Oct 2;16(10):e1008882. doi: 10.1371/journal.ppat.1008882. eCollection 2020 Oct. PLoS Pathog. 2020. PMID: 33007046 Free PMC article.
-
Importance of inhibition of binding of complement factor H for serum bactericidal antibody responses to meningococcal factor H-binding protein vaccines.J Infect Dis. 2013 Aug 15;208(4):627-36. doi: 10.1093/infdis/jit239. Epub 2013 May 28. J Infect Dis. 2013. PMID: 23715659 Free PMC article.
-
Effect of complement Factor H on anti-FHbp serum bactericidal antibody responses of infant rhesus macaques boosted with a licensed meningococcal serogroup B vaccine.Vaccine. 2015 Dec 16;33(51):7168-7175. doi: 10.1016/j.vaccine.2015.10.135. Epub 2015 Nov 10. Vaccine. 2015. PMID: 26562320 Free PMC article.
-
Exploring the Ability of Meningococcal Vaccines to Elicit Mucosal Immunity: Insights from Humans and Mice.Pathogens. 2021 Jul 18;10(7):906. doi: 10.3390/pathogens10070906. Pathogens. 2021. PMID: 34358056 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

