Representation of non-spatial and spatial information in the lateral entorhinal cortex
- PMID: 22065409
- PMCID: PMC3203372
- DOI: 10.3389/fnbeh.2011.00069
Representation of non-spatial and spatial information in the lateral entorhinal cortex
Abstract
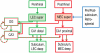
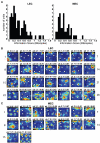
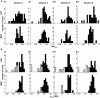
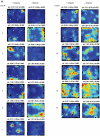
Some theories of memory propose that the hippocampus integrates the individual items and events of experience within a contextual or spatial framework. The hippocampus receives cortical input from two major pathways: the medial entorhinal cortex (MEC) and the lateral entorhinal cortex (LEC). During exploration in an open field, the firing fields of MEC grid cells form a periodically repeating, triangular array. In contrast, LEC neurons show little spatial selectivity, and it has been proposed that the LEC may provide non-spatial input to the hippocampus. Here, we recorded MEC and LEC neurons while rats explored an open field that contained discrete objects. LEC cells fired selectively at locations relative to the objects, whereas MEC cells were weakly influenced by the objects. These results provide the first direct demonstration of a double dissociation between LEC and MEC inputs to the hippocampus under conditions of exploration typically used to study hippocampal place cells.
Keywords: grid cells; hippocampus; medial entorhinal cortex; memory; navigation; objects.
Figures





















References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

