Heat shock protein 70 kDa chaperone/DnaJ cochaperone complex employs an unusual dynamic interface
- PMID: 22065753
- PMCID: PMC3223468
- DOI: 10.1073/pnas.1111220108
Heat shock protein 70 kDa chaperone/DnaJ cochaperone complex employs an unusual dynamic interface
Abstract
The heat shock protein 70 kDa (Hsp70)/DnaJ/nucleotide exchange factor system assists in intracellular protein (re)folding. Using solution NMR, we obtained a three-dimensional structure for a 75-kDa Hsp70-DnaJ complex in the ADP state, loaded with substrate peptide. We establish that the J domain (residues 1-70) binds with its positively charged helix II to a negatively charged loop in the Hsp70 nucleotide-binding domain. The complex shows an unusual "tethered" binding mode which is stoichiometric and saturable, but which has a dynamic interface. The complex represents part of a triple complex of Hsp70 and DnaJ both bound to substrate protein. Mutagenesis data indicate that the interface is also of relevance for the interaction of Hsp70 and DnaJ in the ATP state. The solution complex is completely different from a crystal structure of a disulfide-linked complex of homologous proteins [Jiang, et al. (2007) Mol Cell 28:422-433].
Conflict of interest statement
The authors declare no conflict of interest.
Figures


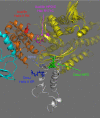
Comment in
-
Evaluation of competing J domain:Hsp70 complex models in light of existing mutational and NMR data.Proc Natl Acad Sci U S A. 2012 Mar 27;109(13):E734; author reply E735. doi: 10.1073/pnas.1120597109. Epub 2012 Mar 7. Proc Natl Acad Sci U S A. 2012. PMID: 22403069 Free PMC article. No abstract available.
References
-
- Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125:443–451. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

