Femtosecond dynamics coupled to chemical barrier crossing in a Born-Oppenheimer enzyme
- PMID: 22065757
- PMCID: PMC3219149
- DOI: 10.1073/pnas.1114900108
Femtosecond dynamics coupled to chemical barrier crossing in a Born-Oppenheimer enzyme
Abstract
Contributions of fast (femtosecond) dynamic motion to barrier crossing at enzyme catalytic sites is in dispute. Human purine nucleoside phosphorylase (PNP) forms a ribocation-like transition state in the phosphorolysis of purine nucleosides and fast protein motions have been proposed to participate in barrier crossing. In the present study, (13)C-, (15)N-, (2)H-labeled human PNP (heavy PNP) was expressed, purified to homogeneity, and shown to exhibit a 9.9% increase in molecular mass relative to its unlabeled counterpart (light PNP). Kinetic isotope effects and steady-state kinetic parameters were indistinguishable for both enzymes, indicating that transition-state structure, equilibrium binding steps, and the rate of product release were not affected by increased protein mass. Single-turnover rate constants were slowed for heavy PNP, demonstrating reduced probability of chemical barrier crossing from enzyme-bound substrates to enzyme-bound products. In a second, independent method to probe barrier crossing, heavy PNP exhibited decreased forward commitment factors, also revealing mass-dependent decreased probability for barrier crossing. Increased atomic mass in human PNP alters bond vibrational modes on the femtosecond time scale and reduces on-enzyme chemical barrier crossing. This study demonstrates coupling of enzymatic bond vibrations on the femtosecond time scale to barrier crossing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
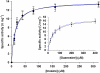
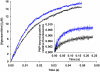
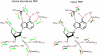
 where ν, k and μ are frequency, force constant and reduced mass, respectively. Likewise, the vibrational energy levels,
where ν, k and μ are frequency, force constant and reduced mass, respectively. Likewise, the vibrational energy levels,  , where E, n and h are energy, quantum number and Planck’s constant, respectively, are lowered by increased protein mass. The shorter arrows shown for heavy PNP illustrate the altered vibrational property. Green arrows promote barrier crossing and red arrows are orthogonal to the reaction coordinate. Heavy PNP has a reduced probability of reaching (and crossing) the transition state. The change is manifested in the experimentally observed decrease in rate of on-enzyme chemistry for heavy PNP. The diagram is modified from (45) and the amino acid contacts are from (46).
, where E, n and h are energy, quantum number and Planck’s constant, respectively, are lowered by increased protein mass. The shorter arrows shown for heavy PNP illustrate the altered vibrational property. Green arrows promote barrier crossing and red arrows are orthogonal to the reaction coordinate. Heavy PNP has a reduced probability of reaching (and crossing) the transition state. The change is manifested in the experimentally observed decrease in rate of on-enzyme chemistry for heavy PNP. The diagram is modified from (45) and the amino acid contacts are from (46).References
-
- Hammes-Schiffer S, Benkovic SJ. Relating protein motion to catalysis. Annu Rev Biochem. 2006;75:519–541. - PubMed
-
- Benkovic SJ, Hammes-Schiffer S. A perspective on enzyme catalysis. Science. 2003;301:1196–1202. - PubMed
-
- Klinman JP. Enzyme dynamics: control of active-site compression. Nat Chem Biol. 2010;2:907–909. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

