Sulforaphane induces antioxidative and antiproliferative responses by generating reactive oxygen species in human bronchial epithelial BEAS-2B cells
- PMID: 22065904
- PMCID: PMC3207051
- DOI: 10.3346/jkms.2011.26.11.1474
Sulforaphane induces antioxidative and antiproliferative responses by generating reactive oxygen species in human bronchial epithelial BEAS-2B cells
Abstract
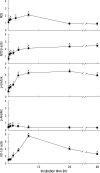
Sulforaphane (SFN) is a naturally occurring compound which is known to induce the phase II antioxidant genes via Nrf2 activation, although the underlying mechanism has not been fully elucidated. In this study, we investigated Nrf2 induction in response to SFN in human bronchial epithelial BEAS-2B cells and determined the signaling pathways involved in this process. SFN treatment reduced cell viability. Prior to cell death, intracellular reactive oxygen species (ROS) were generated at a high rate within a minute of commencing SFN treatment. Pretreatment with antioxidant N-acetylcysteine (NAC) blocked SFN-induced decrease in cell growth. Erk1/2 was activated within 30 min of SFN addition, whereas Akt phosphorylation did not significantly change until the first 8 hr after SFN treatment but then became substantially low until 48 hr. Inhibition of Erk1/2 phosphorylation attenuated SFN-induced loss of cell viability. Nrf2 protein levels in both nuclear and whole cell lysates were increased by SFN treatment, which was dependent on ROS production. Knockdown of Nrf2 with siRNA attenuated SFN-induced heme oxygenase-1 (HO-1) up-regulation. Induction of the Nrf2/HO-1 after SFN treatment was potently suppressed by pretreatment with NAC. Overall, our results indicate that SFN mediates antioxidative and antiproliferative responses by generating ROS in BEAS-2B cells.
Keywords: BEAS-2B Cells; Heme Oxygenase-1; Nrf2; Oxidative Stress; Reactive Oxygen Species; Sulforaphane.
Figures
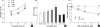

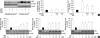
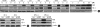

Similar articles
-
Reactive oxygen species and PI3K/Akt signaling play key roles in the induction of Nrf2-driven heme oxygenase-1 expression in sulforaphane-treated human mesothelioma MSTO-211H cells.Food Chem Toxicol. 2012 Feb;50(2):116-23. doi: 10.1016/j.fct.2011.10.035. Epub 2011 Oct 14. Food Chem Toxicol. 2012. PMID: 22019695
-
Bilirubin mediated oxidative stress involves antioxidant response activation via Nrf2 pathway.Cell Signal. 2014 Mar;26(3):512-20. doi: 10.1016/j.cellsig.2013.11.029. Epub 2013 Dec 2. Cell Signal. 2014. PMID: 24308969
-
Effects of antioxidants on oxidative stress and inflammatory responses of human bronchial epithelial cells exposed to particulate matter and cigarette smoke extract.Toxicol In Vitro. 2020 Sep;67:104883. doi: 10.1016/j.tiv.2020.104883. Epub 2020 May 6. Toxicol In Vitro. 2020. PMID: 32387680
-
Modulation of mitochondrial functions by the indirect antioxidant sulforaphane: a seemingly contradictory dual role and an integrative hypothesis.Free Radic Biol Med. 2013 Dec;65:1078-1089. doi: 10.1016/j.freeradbiomed.2013.08.182. Epub 2013 Aug 30. Free Radic Biol Med. 2013. PMID: 23999506 Review.
-
Multi-targeted prevention of cancer by sulforaphane.Cancer Lett. 2008 Oct 8;269(2):291-304. doi: 10.1016/j.canlet.2008.04.018. Epub 2008 May 27. Cancer Lett. 2008. PMID: 18504070 Free PMC article. Review.
Cited by
-
An Evaluation of the Anti-Carcinogenic Response of Major Isothiocyanates in Non-Metastatic and Metastatic Melanoma Cells.Antioxidants (Basel). 2021 Feb 13;10(2):284. doi: 10.3390/antiox10020284. Antioxidants (Basel). 2021. PMID: 33668498 Free PMC article.
-
Sulforaphane and Other Nutrigenomic Nrf2 Activators: Can the Clinician's Expectation Be Matched by the Reality?Oxid Med Cell Longev. 2016;2016:7857186. doi: 10.1155/2016/7857186. Epub 2016 Jan 6. Oxid Med Cell Longev. 2016. PMID: 26881038 Free PMC article. Review.
-
Sulforaphane reactivates cellular antioxidant defense by inducing Nrf2/ARE/Prdx6 activity during aging and oxidative stress.Sci Rep. 2017 Oct 26;7(1):14130. doi: 10.1038/s41598-017-14520-8. Sci Rep. 2017. PMID: 29074861 Free PMC article.
-
Modulation of apoptosis by sulforaphane is associated with PGC-1α stimulation and decreased oxidative stress in cardiac myoblasts.Mol Cell Biochem. 2015 Mar;401(1-2):61-70. doi: 10.1007/s11010-014-2292-z. Epub 2014 Dec 7. Mol Cell Biochem. 2015. PMID: 25481685
-
Precision nutrition to reset virus-induced human metabolic reprogramming and dysregulation (HMRD) in long-COVID.NPJ Sci Food. 2024 Mar 30;8(1):19. doi: 10.1038/s41538-024-00261-2. NPJ Sci Food. 2024. PMID: 38555403 Free PMC article. Review.
References
-
- Lund E. Non-nutritive bioactive constituents of plants: dietary sources and health benefits of glucosinolates. Int J Vitam Nutr Res. 2003;73:135–143. - PubMed
-
- Kushad MM, Brown AF, Kurilich AC, Juvik JA, Klein BP, Wallig MA, Jeffery EH. Variation of glucosinolates in vegetable crops of Brassica oleracea. J Agric Food Chem. 1999;47:1541–1548. - PubMed
-
- Chuang LT, Moqattash ST, Gretz HF, Nezhat F, Rahaman J, Chiao JW. Sulforaphane induces growth arrest and apoptosis in human ovarian cancer cells. Acta Obstet Gynecol Scand. 2007;86:1263–1268. - PubMed
-
- Matsui TA, Murata H, Sakabe T, Sowa Y, Horie N, Nakanishi R, Sakai T, Kubo T. Sulforaphane induces cell cycle arrest and apoptosis in murine osteosarcoma cells in vitro and inhibits tumor growth in vivo. Oncol Rep. 2007;18:1263–1268. - PubMed
-
- Conaway CC, Wang CX, Pittman B, Yang YM, Schwartz JE, Tian D, McIntee EJ, Hecht SS, Chung FL. Phenethyl isothiocyanate and sulforaphane and their N-acetylcysteine conjugates inhibit malignant progression of lung adenomas induced by tobacco carcinogens in A/J mice. Cancer Res. 2005;65:8548–8557. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

