Anatomical pathways involved in generating and sensing rhythmic whisker movements
- PMID: 22065951
- PMCID: PMC3207327
- DOI: 10.3389/fnint.2011.00053
Anatomical pathways involved in generating and sensing rhythmic whisker movements
Abstract
The rodent whisker system is widely used as a model system for investigating sensorimotor integration, neural mechanisms of complex cognitive tasks, neural development, and robotics. The whisker pathways to the barrel cortex have received considerable attention. However, many subcortical structures are paramount to the whisker system. They contribute to important processes, like filtering out salient features, integration with other senses, and adaptation of the whisker system to the general behavioral state of the animal. We present here an overview of the brain regions and their connections involved in the whisker system. We do not only describe the anatomy and functional roles of the cerebral cortex, but also those of subcortical structures like the striatum, superior colliculus, cerebellum, pontomedullary reticular formation, zona incerta, and anterior pretectal nucleus as well as those of level setting systems like the cholinergic, histaminergic, serotonergic, and noradrenergic pathways. We conclude by discussing how these brain regions may affect each other and how they together may control the precise timing of whisker movements and coordinate whisker perception.
Keywords: anatomy; barrel cortex; basal ganglia; cerebellum; follicle–sinus complex; rhythmic movements; sensorimotor integration; vibrissa.
Figures


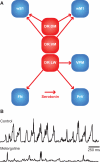
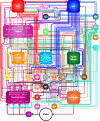
References
-
- Ahl A. S. (1982). Evidence of use of vibrissae in swimming in Sigmodon fulviventer. Anim. Behav. 30, 1203–1206 10.1016/S0003-3472(82)80211-X - DOI
LinkOut - more resources
Full Text Sources

