Improved spinal fusion efficacy by long-term delivery of bone morphogenetic protein-2 in a rabbit model
- PMID: 22066556
- PMCID: PMC3247898
- DOI: 10.3109/17453674.2011.636675
Improved spinal fusion efficacy by long-term delivery of bone morphogenetic protein-2 in a rabbit model
Abstract
Background and purpose: Various new delivery systems for recombinant human bone morphogenetic protein-2 (rhBMP-2) have been introduced to improve its efficacy in osteogenesis. Of these, we have previously developed heparin-conjugated PLGA nanospheres (HCPN) as a long-term delivery system for BMP-2. In vitro studies have shown that the BMP-2 long-term delivery system enhances the level of bone formation. However, the long-term effects of BMP-2 on spinal fusion have not been assessed. Therefore, we now tested the hypothesis that the long-term delivery of BMP-2 using HCPN improves spinal fusion compared to short-term delivery in a rabbit fusion model.
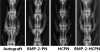
Methods: 24 adult New Zealand White rabbits underwent posterolateral fusion (6 animals in 4 groups). The autograft group received an autologous iliac chip bone graft as a positive control. The BMP-2-PN group received rhBMP-2 (20 μg per implant) and PLGA nanospheres (PN) suspended in fibrin gel, and served as a short-term release group. The HCPN group received HCPN suspended in fibrin gel without BMP-2 as a negative control. The BMP-2-HCPN group received rhBMP-2 (20 μg per implant)-bound HCPN suspended in fibrin gel and served as a long-term release group. All animals were killed 12 weeks after surgery. Manual palpation, axial tensile tests, radiography, and histological evaluations were then performed.
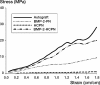
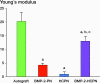
Results: The spinal fusion rate and Young's modulus of the fusion mass were better in the BMP-2 long-term delivery group than in the short-term delivery group at an equivalent dose. However, the outcome of the long-term delivery was inferior to that of the autograft group.
Interpretation: The HCPN system showed potential as an effective carrier that might improve the osteogenic efficacy of BMP-2 for spinal fusion.
Figures
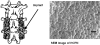
References
-
- Akamaru T, Suh D, Boden SD, Kim HS, Minamide A, Louis-Ugbo J. Simple carrier matrix modifications can enhance delivery of recombinant human bone morphogenetic protein-2 for posterolateral spine fusion. Spine. 2003;28(5):429–34. - PubMed
-
- Benglis D, Wang MY, Levi AD. A comprehensive review of the safety profile of bone morphogenetic protein in spine surgery. Neurosurgery. 2008;((Suppl 2)):62. (5):ONS423-31; discussion ONS31. - PubMed
-
- Boden SD, Schimandle JH, Hutton WC. Lumbar intertransverse-process spinal arthrodesis with use of a bovine bone-derived osteoinductive protein. J Bone Joint Surg (Am) 1995;77(9):1404–17. - PubMed
-
- Burkus JK, Gornet MF, Dickman CA, Zdeblick TA. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech. 2002;15(5):337–49. - PubMed
-
- Dimar JR, Glassman SD, Burkus KJ, Carreon LY. Clinical outcomes and fusion success at 2 years of single-level instrumented posterolateral fusions with recombinant human bone morphogenetic protein-2/compression resistant matrix versus iliac crest bone graft. Spine. 2006;31(22):2534–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
