Investigating circular dorsal ruffles through varying substrate stiffness and mathematical modeling
- PMID: 22067149
- PMCID: PMC3207170
- DOI: 10.1016/j.bpj.2011.09.047
Investigating circular dorsal ruffles through varying substrate stiffness and mathematical modeling
Abstract
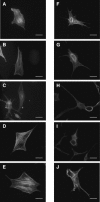
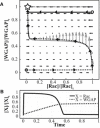
Circular dorsal ruffles (CDRs) are transient actin-rich ringlike structures that form on the dorsal surface of growth-factor stimulated cells. However, the dynamics and mechanism of formation of CDRs are still unknown. It has been observed that CDR formation leads to stress fibers disappearing near the CDRs. Because stress fiber formation can be modified by substrate stiffness, we examined the effect of substrate stiffness on CDR formation by seeding NIH 3T3 fibroblasts on glass and polydimethylsiloxane substrates of varying stiffnesses from 20 kPa to 1800 kPa. We found that increasing substrate stiffness increased the lifetime of the CDRs. We developed a mathematical model of the signaling pathways involved in CDR formation to provide insight into this lifetime and size dependence that is linked to substrate stiffness via Rac-Rho antagonism. From the model, increasing stiffness raised mDia1-nucleated stress fiber formation due to Rho activation. The increased stress fibers present increased replenishment of the G-actin pool, therefore prolonging Arp2/3-nucleated CDR formation due to Rac activation. Negative feedback by WAVE-related RacGAP on Rac explained how CDR actin propagates as an excitable wave, much like wave propagation in other excitable medium, e.g., nerve signal transmission.
Copyright © 2011 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Mellström K., Heldin C.H., Westermark B. Induction of circular membrane ruffling on human fibroblasts by platelet-derived growth factor. Exp. Cell Res. 1988;177:347–359. - PubMed
-
- Mellstroöm K., Höglund A.S., Lindberg U. The effect of platelet-derived growth factor on morphology and motility of human glial cells. J. Muscle Res. Cell Motil. 1983;4:589–609. - PubMed
-
- Tamura M., Iwamoto Y. The effect of platelet-derived growth factor on phagocytosis of cultured human trabecular cells. Exp. Eye Res. 1989;48:761–770. - PubMed
-
- Araki N., Egami Y., Hatae T. Phosphoinositide metabolism during membrane ruffling and macropinosome formation in EGF-stimulated A431 cells. Exp. Cell Res. 2007;313:1496–1507. - PubMed
-
- Arvidsson A.K., Heldin C.H., Claesson-Welsh L. Transduction of circular membrane ruffling by the platelet-derived growth factor β-receptor is dependent on its kinase insert. Cell Growth Diff. 1992;3:881–887. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

