Base-flipping mechanism in postmismatch recognition by MutS
- PMID: 22067162
- PMCID: PMC3207177
- DOI: 10.1016/j.bpj.2011.09.045
Base-flipping mechanism in postmismatch recognition by MutS
Abstract
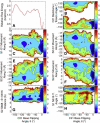
DNA mismatch recognition and repair is vital for preserving the fidelity of the genome. Conserved across prokaryotes and eukaryotes, MutS is the primary protein that is responsible for recognizing a variety of DNA mismatches. From molecular dynamics simulations of the Escherichia coli MutS-DNA complex, we describe significant conformational dynamics in the DNA surrounding a G·T mismatch that involves weakening of the basepair hydrogen bonding in the basepair adjacent to the mismatch and, in one simulation, complete base opening via the major groove. The energetics of base flipping was further examined with Hamiltonian replica exchange free energy calculations revealing a stable flipped-out state with an initial barrier of ~2 kcal/mol. Furthermore, we observe changes in the local DNA structure as well as in the MutS structure that appear to be correlated with base flipping. Our results suggest a role of base flipping as part of the repair initiation mechanism most likely leading to sliding-clamp formation.
Copyright © 2011 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
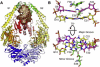

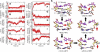
Similar articles
-
Deciphering the mismatch recognition cycle in MutS and MSH2-MSH6 using normal-mode analysis.Biophys J. 2009 Mar 4;96(5):1707-20. doi: 10.1016/j.bpj.2008.10.071. Biophys J. 2009. PMID: 19254532 Free PMC article.
-
Importance of base-pair opening for mismatch recognition.Nucleic Acids Res. 2020 Nov 18;48(20):11322-11334. doi: 10.1093/nar/gkaa896. Nucleic Acids Res. 2020. PMID: 33080020 Free PMC article.
-
Mechanism for verification of mismatched and homoduplex DNAs by nucleotides-bound MutS analyzed by molecular dynamics simulations.Proteins. 2016 Sep;84(9):1287-303. doi: 10.1002/prot.25077. Epub 2016 Jun 15. Proteins. 2016. PMID: 27238299
-
Structure and function of mismatch repair proteins.Mutat Res. 2000 Aug 30;460(3-4):245-56. doi: 10.1016/s0921-8777(00)00030-6. Mutat Res. 2000. PMID: 10946232 Review.
-
DNA mismatch repair: MutS structures bound to mismatches.Curr Opin Struct Biol. 2001 Feb;11(1):47-52. doi: 10.1016/s0959-440x(00)00169-x. Curr Opin Struct Biol. 2001. PMID: 11179891 Review.
Cited by
-
Protein Loop Conformational Free Energy Changes via an Alchemical Path without Reaction Coordinates.J Phys Chem Lett. 2021 May 13;12(18):4368-4377. doi: 10.1021/acs.jpclett.1c00778. Epub 2021 May 3. J Phys Chem Lett. 2021. PMID: 33938761 Free PMC article.
-
Single gold-bridged nanoprobes for identification of single point DNA mutations.Nat Commun. 2019 Feb 19;10(1):836. doi: 10.1038/s41467-019-08769-y. Nat Commun. 2019. PMID: 30783107 Free PMC article.
-
Recent advances in transferable coarse-grained modeling of proteins.Adv Protein Chem Struct Biol. 2014;96:143-80. doi: 10.1016/bs.apcsb.2014.06.005. Epub 2014 Aug 24. Adv Protein Chem Struct Biol. 2014. PMID: 25443957 Free PMC article. Review.
-
Conformational Free Energy Changes via an Alchemical Path without Reaction Coordinates.J Phys Chem Lett. 2018 Aug 2;9(15):4428-4435. doi: 10.1021/acs.jpclett.8b01851. Epub 2018 Jul 24. J Phys Chem Lett. 2018. PMID: 30024165 Free PMC article.
-
The 'very moment' when UDG recognizes a flipped-out uracil base in dsDNA.Sci Rep. 2025 Mar 7;15(1):7993. doi: 10.1038/s41598-025-91705-6. Sci Rep. 2025. PMID: 40055399 Free PMC article.
References
-
- Lahue R.S., Au K.G., Modrich P. DNA mismatch correction in a defined system. Science. 1989;245:160–164. - PubMed
-
- Grilley M., Griffith J., Modrich P. Bidirectional excision in methyl-directed mismatch repair. J. Biol. Chem. 1993;268:11830–11837. - PubMed
-
- Cooper D.L., Lahue R.S., Modrich P. Methyl-directed mismatch repair is bidirectional. J. Biol. Chem. 1993;268:11823–11829. - PubMed
-
- Au K.G., Welsh K., Modrich P. Initiation of methyl-directed mismatch repair. J. Biol. Chem. 1992;267:12142–12148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

