Neurological diseases and pain
- PMID: 22067541
- PMCID: PMC3281476
- DOI: 10.1093/brain/awr271
Neurological diseases and pain
Abstract
Chronic pain is a frequent component of many neurological disorders, affecting 20-40% of patients for many primary neurological diseases. These diseases result from a wide range of pathophysiologies including traumatic injury to the central nervous system, neurodegeneration and neuroinflammation, and exploring the aetiology of pain in these disorders is an opportunity to achieve new insight into pain processing. Whether pain originates in the central or peripheral nervous system, it frequently becomes centralized through maladaptive responses within the central nervous system that can profoundly alter brain systems and thereby behaviour (e.g. depression). Chronic pain should thus be considered a brain disease in which alterations in neural networks affect multiple aspects of brain function, structure and chemistry. The study and treatment of this disease is greatly complicated by the lack of objective measures for either the symptoms or the underlying mechanisms of chronic pain. In pain associated with neurological disease, it is sometimes difficult to obtain even a subjective evaluation of pain, as is the case for patients in a vegetative state or end-stage Alzheimer's disease. It is critical that neurologists become more involved in chronic pain treatment and research (already significant in the fields of migraine and peripheral neuropathies). To achieve this goal, greater efforts are needed to enhance training for neurologists in pain treatment and promote greater interest in the field. This review describes examples of pain in different neurological diseases including primary neurological pain conditions, discusses the therapeutic potential of brain-targeted therapies and highlights the need for objective measures of pain.
Figures

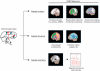
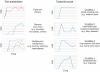
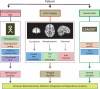
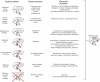
References
-
- Abbruzzese G, Berardelli A. Sensorimotor integration in movement disorders. Mov Disord. 2003;18:231–40. - PubMed
-
- Aghakhani N, Parker F, David P, Morar S, Lacroix C, Benoudiba F, et al. Long-term follow-up of Chiari-related syringomyelia in adults: analysis of 157 surgically treated cases. Neurosurgery. 2009;64:308–15. - PubMed
-
- Albano B, Dinia L, Del Sette M, Gandolfo C, Sivori G, Finocchi C. Fabry disease in patients with migraine with aura. Neurol Sci. 2010;31(Suppl 1):S167–9. - PubMed
-
- Albin RL, Young AB. Somatosensory phenomena in Huntington's disease. Mov Disord. 1988;3:343–6. - PubMed
-
- Alderfer BS, Arciniegas DB, Silver JM. Treatment of depression following traumatic brain injury. J Head Trauma Rehabil. 2005;20:544–62. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

