The virulence regulator Agr controls the staphylococcal capacity to activate human neutrophils via the formyl peptide receptor 2
- PMID: 22067547
- PMCID: PMC3388272
- DOI: 10.1159/000332142
The virulence regulator Agr controls the staphylococcal capacity to activate human neutrophils via the formyl peptide receptor 2
Abstract
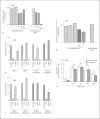
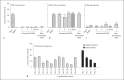
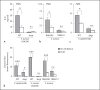
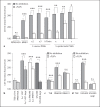
The Agr quorum-sensing system represents the master regulator for staphylococcal virulence factors and is known to have a strong impact on the release of pathogen-associated molecular pattern (PAMP) molecules. Among the various staphylococcal PAMPs, phenol-soluble modulin (PSM) peptides have attracted increasing interest because they are crucial for staphylococcal virulence and have neutrophil-recruiting properties. The latter depend on recognition of PSMs by the neutrophil formyl peptide receptor 2 (FPR2/ALX), for which PSMs are highly efficient agonists. We demonstrate that Agr inactivation in Staphylococcus aureus or S. epidermidis leads to strongly reduced neutrophil responses, which is in agreement with the previously reported strict control of PSM expression by Agr. Agr had a distinct and profound impact on activation of FPR2/ALX but not of the related FPR1 receptor that senses bacterial formylated peptides. S. epidermidis PSMs had similar FPR2/ALX-activating properties but differed in their dependence on N-terminal formylation compared to S. aureus PSMs. Moreover, S. aureus and S. epidermidis PSMs upregulated the neutrophil complement receptor CD11b via FPR2/ALX stimulation in an Agr-dependent fashion. Hence, Agr controls the capacity of staphylococcal pathogens to activate FPR2/ALX-dependent neutrophil responses, underscoring the crucial role of FPR2/ALX and PSMs in staphylococcus-host interaction.
Copyright © 2012 S. Karger AG, Basel.
Figures

References
-
- Otto M, Sussmuth R, Jung G, Gotz F. Structure of the pheromone peptide of the Staphylococcus epidermidis agr system. FEBS Lett. 1998;424:89–94. - PubMed
-
- Ji G, Beavis R, Novick RP. Bacterial interference caused by autoinducing peptide variants. Science. 1997;276:2027–2030. - PubMed
-
- Kong KF, Vuong C, Otto M. Staphylococcus quorum sensing in biofilm formation and infection. Int J Med Microbiol. 2006;296:133–139. - PubMed
-
- Vuong C, Durr M, Carmody AB, Peschel A, Klebanoff SJ, Otto M. Regulated expression of pathogen-associated molecular pattern molecules in Staphylococcus epidermidis: quorum sensing determines pro-inflammatory capacity and production of phenol-soluble modulins. Cell Microbiol. 2004;6:753–759. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

