Maintenance of primary tumor phenotype and genotype in glioblastoma stem cells
- PMID: 22067563
- PMCID: PMC3266381
- DOI: 10.1093/neuonc/nor195
Maintenance of primary tumor phenotype and genotype in glioblastoma stem cells
Abstract
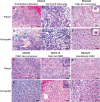
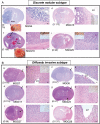
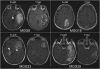
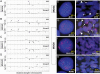
The clinicopathological heterogeneity of glioblastoma (GBM) and the various genetic and phenotypic subtypes in GBM stem cells (GSCs) are well described. However, the relationship between GSCs and the corresponding primary tumor from which they were isolated is poorly understood. We have established GSC-enriched neurosphere cultures from 15 newly diagnosed GBM specimens and examined the relationship between the histopathological and genomic features of GSC-derived orthotopic xenografts and those of the respective patient tumors. GSC-initiated xenografts recapitulate the distinctive cytological hallmarks and diverse histological variants associated with the corresponding patient GBM, including giant cell and gemistocytic GBM, and primitive neuroectodermal tumor (PNET)-like components. This indicates that GSCs generate tumors that preserve patient-specific disease phenotypes. The majority of GSC-derived intracerebral xenografts (11 of 15) demonstrated a highly invasive behavior crossing the midline, whereas the remainder formed discrete nodular and vascular masses. In some cases, GSC invasiveness correlated with preoperative MRI, but not with the status of PI3-kinase/Akt pathways or O(6)-methylguanine methyltransferase expression. Genome-wide screening by array comparative genomic hybridization and fluorescence in situ hybridization revealed that GSCs harbor unique genetic copy number aberrations. GSCs acquiring amplifications of the myc family genes represent only a minority of tumor cells within the original patient tumors. Thus, GSCs are a genetically distinct subpopulation of neoplastic cells within a GBM. These studies highlight the value of GSCs for preclinical modeling of clinically relevant, patient-specific GBM and, thus, pave the way for testing novel anti-GSC/GBM agents for personalized therapy.
Figures

References
-
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi:10.1056/NEJMra0708126. - DOI - PubMed
-
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi:10.1016/S1470-2045(09)70025-7. - DOI - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi:10.1056/NEJMoa043330. - DOI - PubMed
-
- Miller CR, Perry A. Glioblastoma. Arch Pathol Lab Med. 2007;131:397–406. - PubMed
-
- Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi:10.1038/nature07385. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

