Systematic exploration of synergistic drug pairs
- PMID: 22068327
- PMCID: PMC3261710
- DOI: 10.1038/msb.2011.71
Systematic exploration of synergistic drug pairs
Abstract
Drug synergy allows a therapeutic effect to be achieved with lower doses of component drugs. Drug synergy can result when drugs target the products of genes that act in parallel pathways ('specific synergy'). Such cases of drug synergy should tend to correspond to synergistic genetic interaction between the corresponding target genes. Alternatively, 'promiscuous synergy' can arise when one drug non-specifically increases the effects of many other drugs, for example, by increased bioavailability. To assess the relative abundance of these drug synergy types, we examined 200 pairs of antifungal drugs in S. cerevisiae. We found 38 antifungal synergies, 37 of which were novel. While 14 cases of drug synergy corresponded to genetic interaction, 92% of the synergies we discovered involved only six frequently synergistic drugs. Although promiscuity of four drugs can be explained under the bioavailability model, the promiscuity of Tacrolimus and Pentamidine was completely unexpected. While many drug synergies correspond to genetic interactions, the majority of drug synergies appear to result from non-specific promiscuous synergy.
Conflict of interest statement
MC1, HNC, and FPR have filed a US patent application on synergistic antifungal drug combinations discovered in the course of this study.
Figures

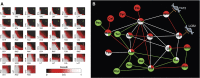


References
-
- Akima H (1970) A new method of interpolation and smooth curve fitting based on local procedures. J ACM 17: 589–602
-
- Baryshnikova A, Costanzo M, Kim Y, Ding H, Koh J, Toufighi K, Youn JY, Ou J, San Luis BJ, Bandyopadhyay S, Hibbs M, Hess D, Gingras AC, Bader GD, Troyanskaya OG, Brown GW, Andrews B, Boone C, Myers CL (2010) Quantitative analysis of fitness and genetic interactions in yeast on a genome scale. Nat Methods 7: 1017–1024 - PMC - PubMed
-
- Berenbaum MC (1989) What is synergy? Pharmacol Rev 41: 93–141 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HG004098/HG/NHGRI NIH HHS/United States
- R21 HG004756/HG/NHGRI NIH HHS/United States
- R01 HG005853/HG/NHGRI NIH HHS/United States
- CA130266/CA/NCI NIH HHS/United States
- GM086115/GM/NIGMS NIH HHS/United States
- R21 MH087394/MH/NIMH NIH HHS/United States
- HG005853-01/HG/NHGRI NIH HHS/United States
- F32 HG004098/HG/NHGRI NIH HHS/United States
- P50 HG004233/HG/NHGRI NIH HHS/United States
- MOP-84305/CAPMC/ CIHR/Canada
- HG004756/HG/NHGRI NIH HHS/United States
- HG003224/HG/NHGRI NIH HHS/United States
- HG004233/HG/NHGRI NIH HHS/United States
- F32 GM086115/GM/NIGMS NIH HHS/United States
- MH087394/MH/NIMH NIH HHS/United States
- R21 CA130266/CA/NCI NIH HHS/United States
- MOP-81340/CAPMC/ CIHR/Canada
- R01 HG003224/HG/NHGRI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

