E/N-cadherin switch mediates cancer progression via TGF-β-induced epithelial-to-mesenchymal transition in extrahepatic cholangiocarcinoma
- PMID: 22068819
- PMCID: PMC3251878
- DOI: 10.1038/bjc.2011.452
E/N-cadherin switch mediates cancer progression via TGF-β-induced epithelial-to-mesenchymal transition in extrahepatic cholangiocarcinoma
Abstract
Background: Epithelial-to-mesenchymal transition (EMT) is a fundamental process governing not only morphogenesis in multicellular organisms, but also cancer progression. During EMT, epithelial cadherin (E-cadherin) is downregulated while neural cadherin (N-cadherin) is upregulated, referred to as 'cadherin switch'. This study aimed to investigate whether cadherin switch promotes cancer progression in cholangiocarcinoma (CC).
Methods: CC cell lines were examined for migration, invasion, and morphological changes with typical EMT-induced model using recombinant TGF-β1. The changes in E-cadherin and N-cadherin expression were investigated during EMT. We also examined E-cadherin and N-cadherin expression in resected specimens from extrahepatic CC patients (n=38), and the associations with clinicopathological factors and survival rates.
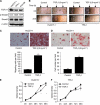
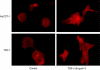
Results: TGF-β1 treatment activated cell migration, invasion, and fibroblastic morphological changes, especially in extrahepatic CC HuCCT-1 cells. These changes occurred with E-cadherin downregulation and N-cadherin upregulation, that is, cadherin switch. Patients with low E-cadherin expression had a significantly lower survival rate than patients with high E-cadherin expression (P=0.0059). Patients with decreasing E-cadherin and increasing N-cadherin expression had a significantly lower survival rate than patients with increasing E-cadherin and decreasing N-cadherin expression (P=0.017).
Conclusion: Cadherin switch promotes cancer progression via TGF-β-induced EMT in extrahepatic CC, suggesting a target for elucidating the mechanisms of invasion and metastasis in extrahepatic CC.
Figures



References
-
- Birchmeier C, Birchmeier W, Brand-Saberi B (1996) Epithelial-mesenchymal transitions in cancer progression. Acta Anat (Basel) 156: 217–226 - PubMed
-
- Chamberlain RS, Blumgart LH (2000) Hilar cholangiocarcinoma: a review and commentary. Ann Surg Oncol 7: 55–66 - PubMed
-
- Christiansen JJ, Rajasekaran AK (2006) Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res 66: 8319–8326 - PubMed
-
- Derycke LD, Bracke ME (2004) N-cadherin in the spotlight of cell-cell adhesion, differentiation, embryogenesis, invasion and signalling. Int J Dev Biol 48: 463–476 - PubMed
-
- Derynck R, Akhurst RJ, Balmain A (2001) TGF-beta signaling in tumor suppression and cancer progression. Nat Genet 29: 117–129 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

